 |
 |
 |
| |
Reduced Darunavir Dose Is as Effective in Maintaining HIV Suppression as the Standard Dose in Virologically Suppressed HIV-Infected Patients. The DRV600 Study
|
| |
| |
Reported by Jules Levin
15th International Workshop on Clinical Pharmacology of HV & Hepatitis Treatment
Washington 19th May 2014
José Molto, Marta Valle, Elena Ferrer, Adrián Curran, José Ramon Santos, Silvana DiYacovo, Pere Domingo, Cristina Miranda, Mar Gutierrez, Bonaventura Clotet, and the DRV600 Study Group.

Program Abstract:
Reduced darunavir dose is as effective in maintaining HIV suppression as the standard dose in virologically suppressed HIV-infected patients.
J. Molto1, M. Valle2, E. Ferrer3, A. Curran4, J.R. Santos1, S. DiYacovo3, P. Domingo5, C. Miranda1, M. Gutiérrez5, B. Clotet6
1Hospital Universitari Germans Trias i Pujol, Fundacio Lluita contra la SIDA, Badalona, Spain;2Institut de Recerca HSCSP-IIB St Pau, PKPD Modeling and Simulation, Barcelona, Spain;3 IDIBELL-Hospital Universitari de Bellvitge, Unitat VIH, Barcelona, Spain; 4Hospital Universitari Vall d'Hebron, Infectious Diseases Department, Barcelona, Spain; 5Hospital de la Santa Creu i Sant Pau, Internal Medicine Department, Barcelona, Spain; 6Hospital Universitari Germans Trias i Pujol, Fundacio IrsiCaixa, Badalona, Spain
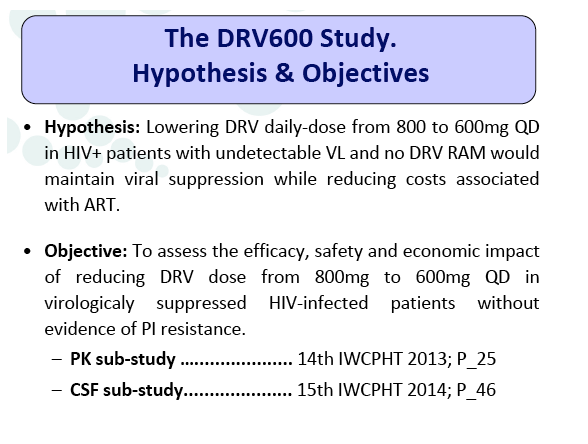
Introduction: The global economic crisis has prompted interest in dose optimization strategies aimed at reducing the cost of antiretroviral treatment (ART) while maintaining its efficacy. The DRV600 study compares the efficacy and safety of a reduced dose with the standard dose of darunavir (DRV) in virologically suppressed HIV-infected patients.
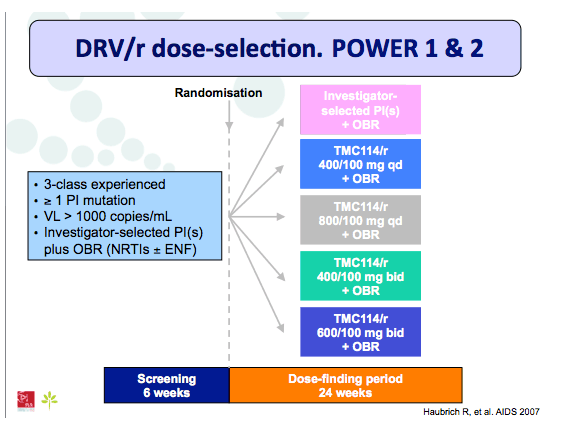
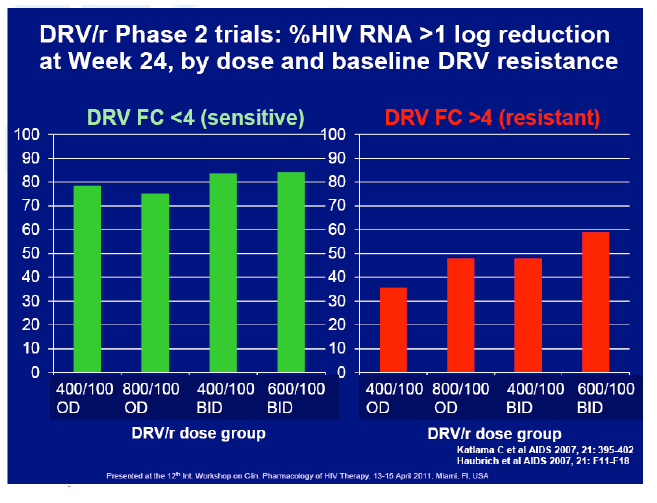
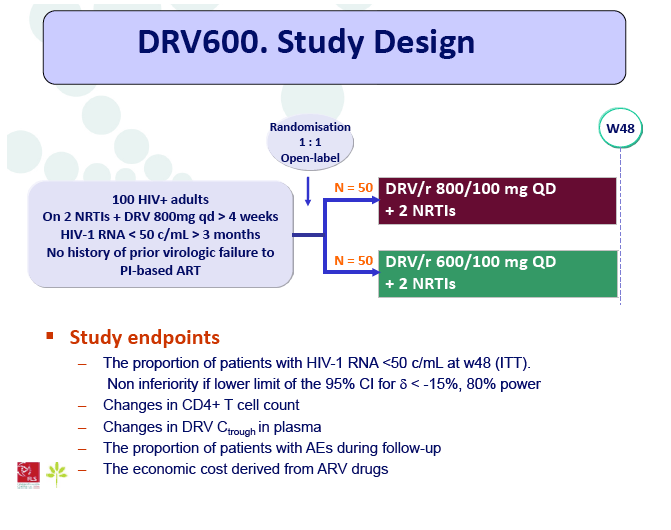
Materials & Methods: The DRV600 study (eudraCT 2011-006272-39) was a multicenter, randomized, open-label clinical trial in HIV-infected patients with a plasma viral load (pVL) <50 copies/mL while on ART including DRV 800mg once daily (QD) plus two nucleos(t)ide reverse transcriptase inhibitors (NRTIs). Documented DRV resistance-associated mutations or prior virologic failure while receiving ART with protease inhibitors were considered exclusion criteria. Participants were randomized either to continue on the standard DRV 800mg QD dose (DRV800) or to reduce the dose to 600mg QD (DRV600). All patients continued receiving ritonavir 100mg QD and the same NRTIs. Treatment failure was defined as two consecutive pVL >50 copies/mL or discontinuation of study treatment by week 48. The trial had 80% power to show non-inferiority for the DRV600 arm (delta=-15%) in the intention-to-treat non-completer=failure (ITT NC=F) population.
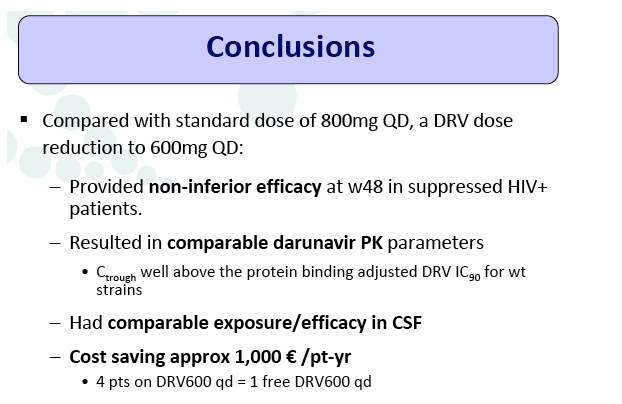
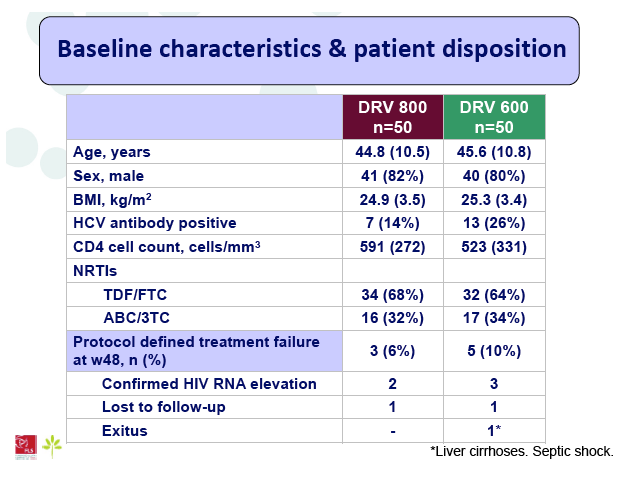
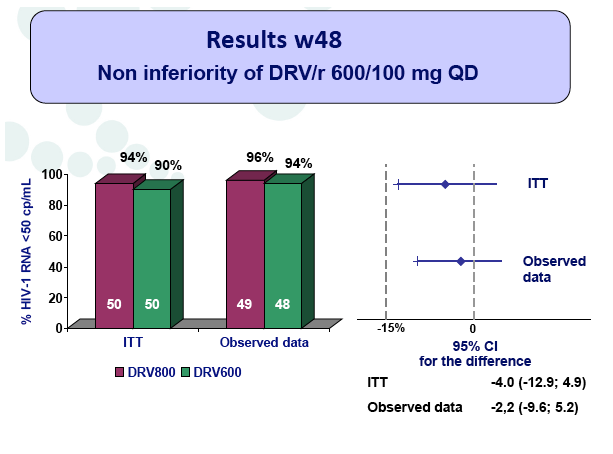
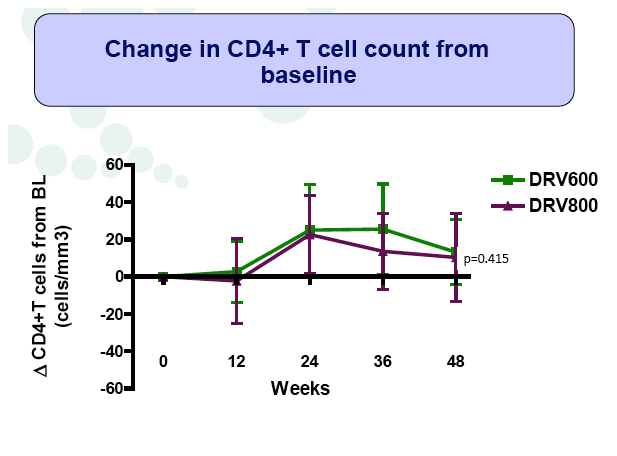
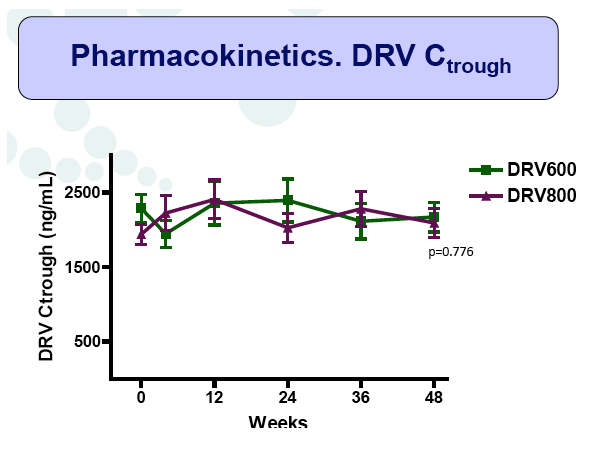
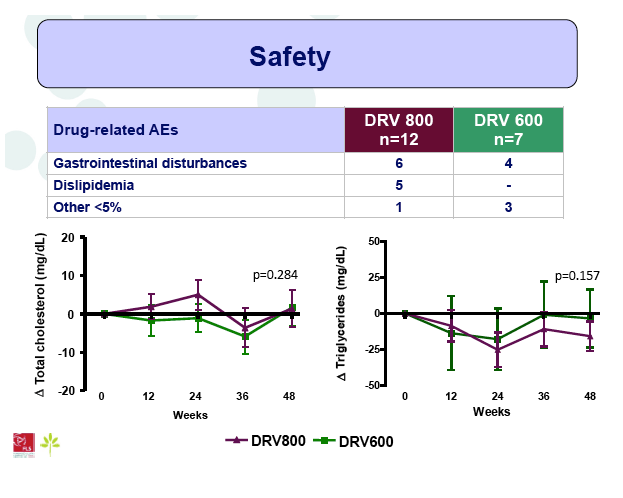
Results: A total of 100 patients were included in the study (DRV800=50; DRV600=50). Of these, 81% were male and 20% were co-infected by HCV. Mean (SD) CD4+ T cell count at baseline was 562 (303) cells/mm3, with no differences between study arms. Two patients from the DRV800 arm and three from the DRV600 arm developed virologic failure by week 48. Additionally, one patient from each arm was lost to follow up and one patient from the DRV600 arm died of septic shock. Thus, the proportion of patients with pVL<50 copies/mL at week 48 in the ITT NC=F analysis was 90% in the DRV600 and 94% in the DRV800 arm (difference -4%; 90% CI: -12.9; 4.9; p=0.46). CD4 cell counts remained stable during follow up in both arms. DRV trough concentrations in plasma were comparable between the two study groups. There was no difference in the frequency of adverse events between study arms.
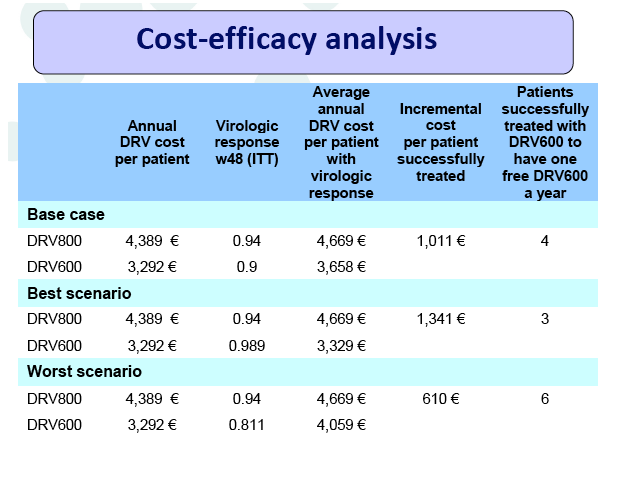
Conclusion: Compared to the standard dose of DRV (800mg QD), a reduced dose (600mg QD) resulted in comparable DRV concentrations in plasma and showed non-inferior virologic efficacy in previously virologically suppressed HIV-infected patients, over 48 weeks. This strategy has the potential to reduce treatment cost of ART and allow more patients to be treated.
|
| |
|
 |
 |
|
|