 |
 |
 |
| |
Pharmacokinetics and Safety of IDX21437, a Novel HCV Nucleotide Prodrug, in Healthy Volunteers and HCV-Infected Subjects: Results of a First-In-Human Study
|
| |
| |
Reported by Jules Levin
15th International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy, Washington, D.C., May 19-21, 2014.
Xiao-Jian Zhou1, Eric Sicard2, Marie-Francoise Temam3, Jie Chen1, Dodie Frank1, Eileen Donovan1, Keith Pietropaolo1, John Z. Sullivan-Bólyai1, Douglas Mayers1
1 Idenix Pharmaceuticals, Inc., Cambridge, MA, USA; 2Algorithme Pharma, Montreal, Canada; 3Idenix Sarl, Montpellier, France
At EASL 2014 last month in London, Idenix reported data on for their nuke IDX21437, similar to sofosbuvir, which they are developing, now starting clinical studies in combination with their NS5A
Idenix Announces Promising Clinical Data and Continued Progress in Nucleotide Prodrug Development Programs for the Treatment of Hepatitis C - (04/07/14)
EASL: Favorable Preclinical Profile of IDX21437, a Novel Uridine Nucleotide Prodrug, for Use in a Direct-Acting Antiviral (DAA) Regimen for HCV - (04/16/14)
EASL: A Phase II Study of Samatasvir (IDX719) in Combination with Simeprevir and Ribavirin in Treatment-Naďve HCV-Infected Subjects with Genotypes 1b and 4 (HELIX-1 Study) - (04/16/14)
EASL: Pharmacokinetic (PK) Drug-Drug Interaction between Samatasvir (IDX719), a Pan-Genotypic NS5A Inhibitor, and Simeprevir in Healthy Volunteers and HCV-Infected Subjects - (04/16/14)
IDX719, HCV NS5A Inhibitor, Demonstrates Pan-Genotypic Activity after Three Days of Monotherapy in Genotype 1, 2, 3 or 4 HCV-Infected
Subjects.....http://www.natap.org/2013/HCV/013113_03.htm
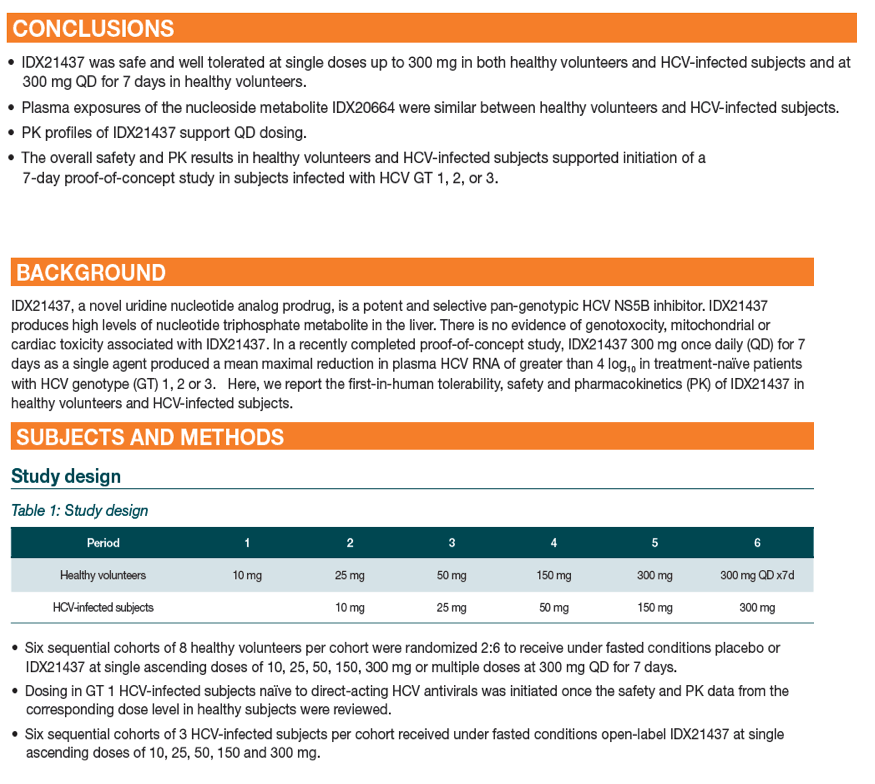
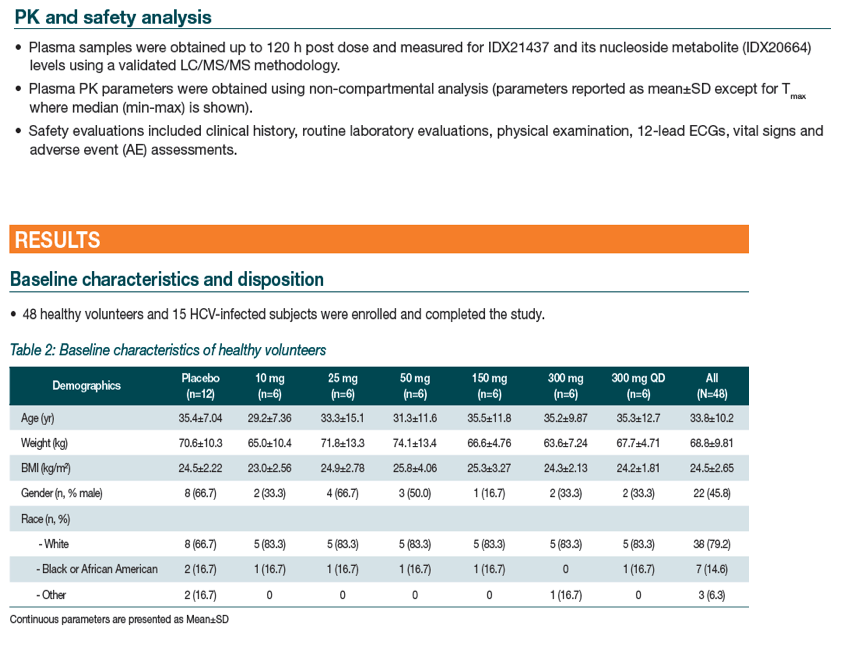
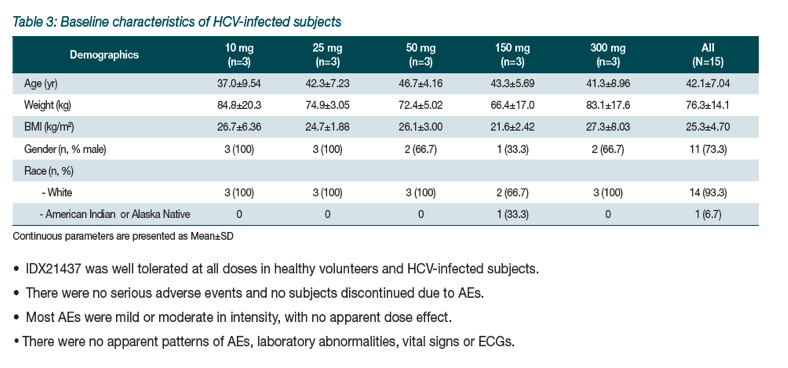
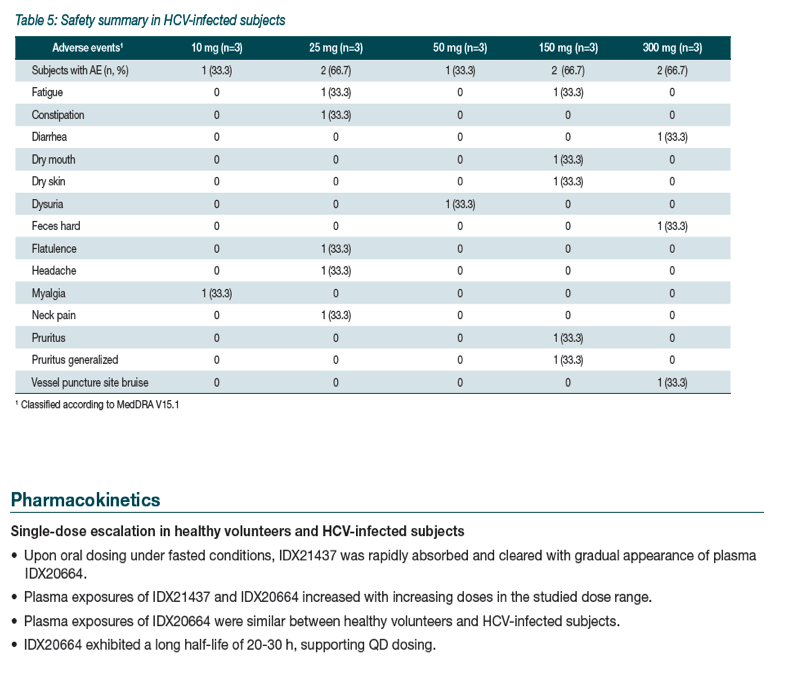
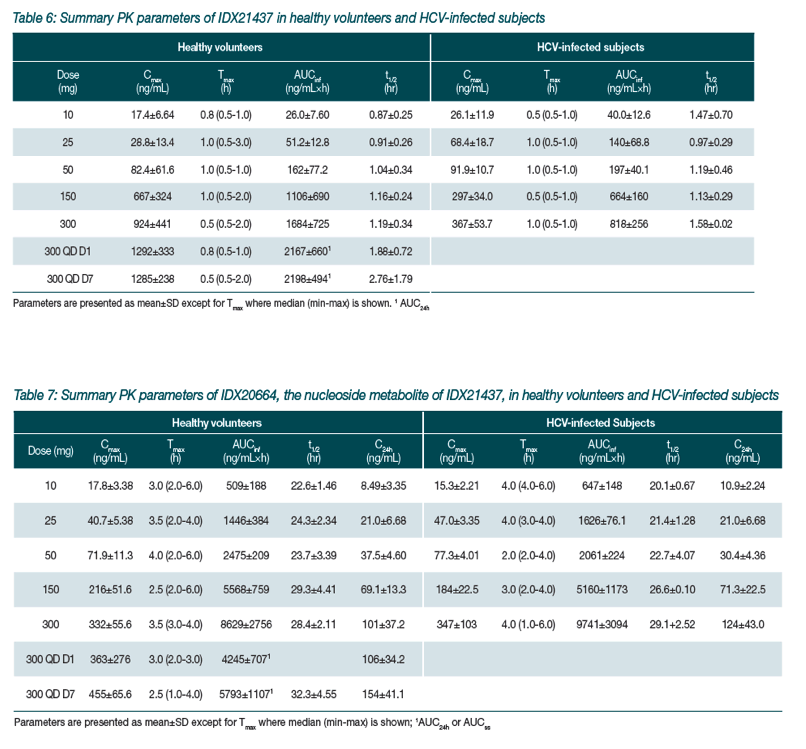
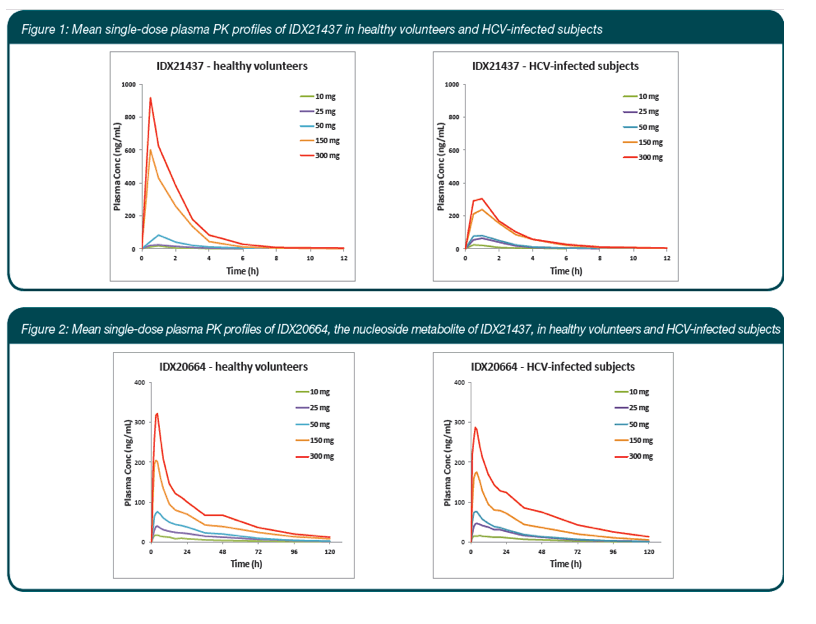
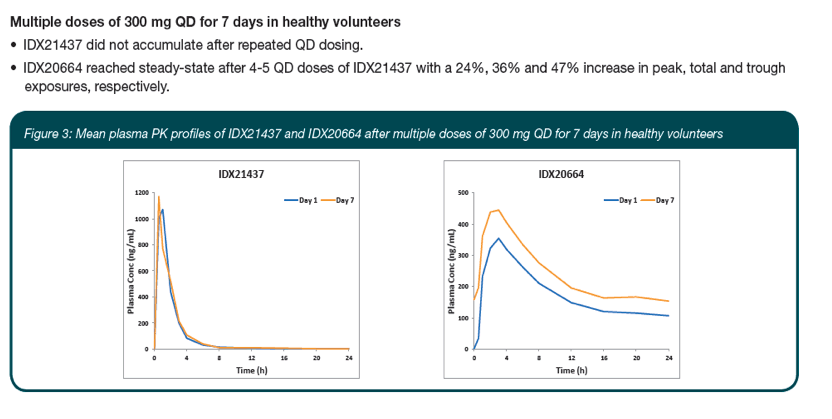
|
| |
|
 |
 |
|
|