 |
 |
 |
| |
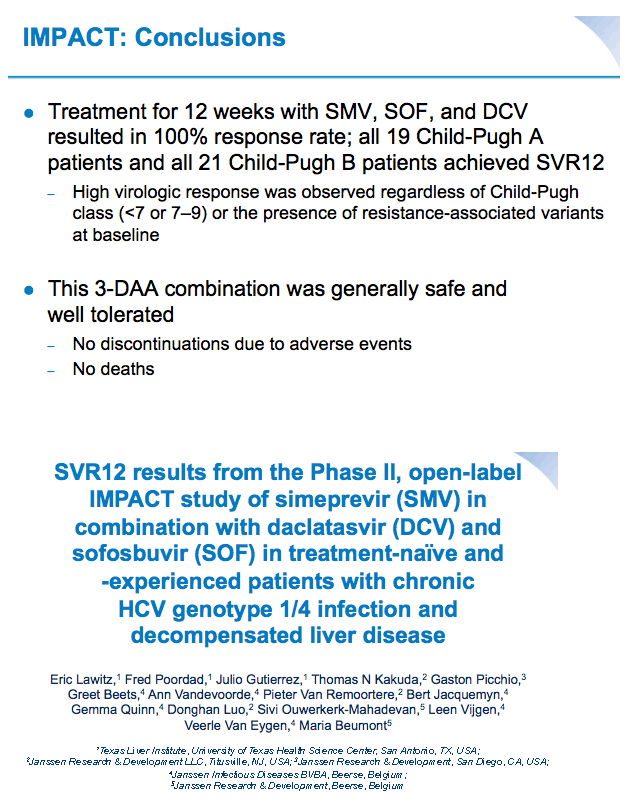
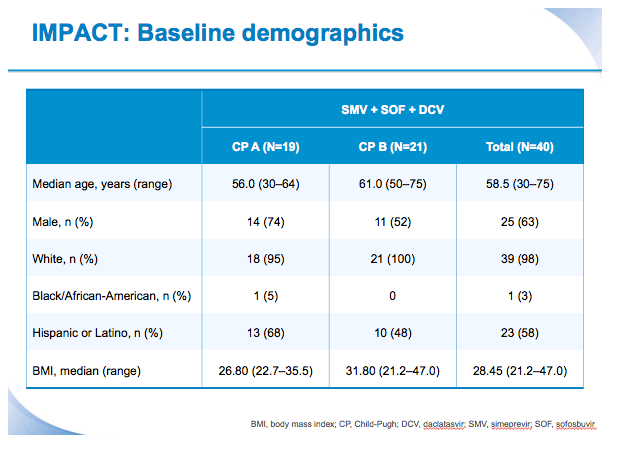
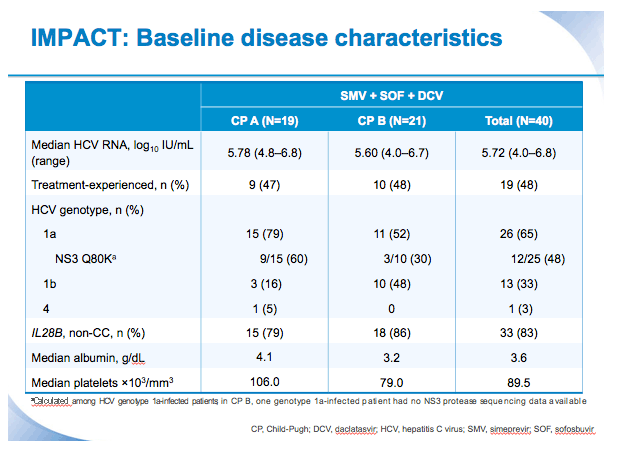
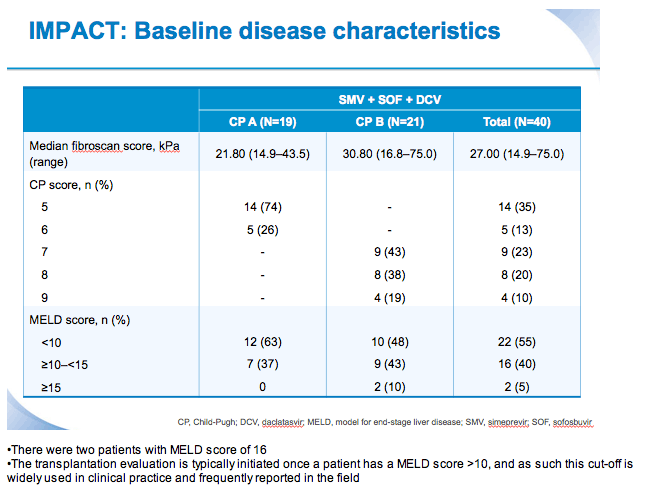
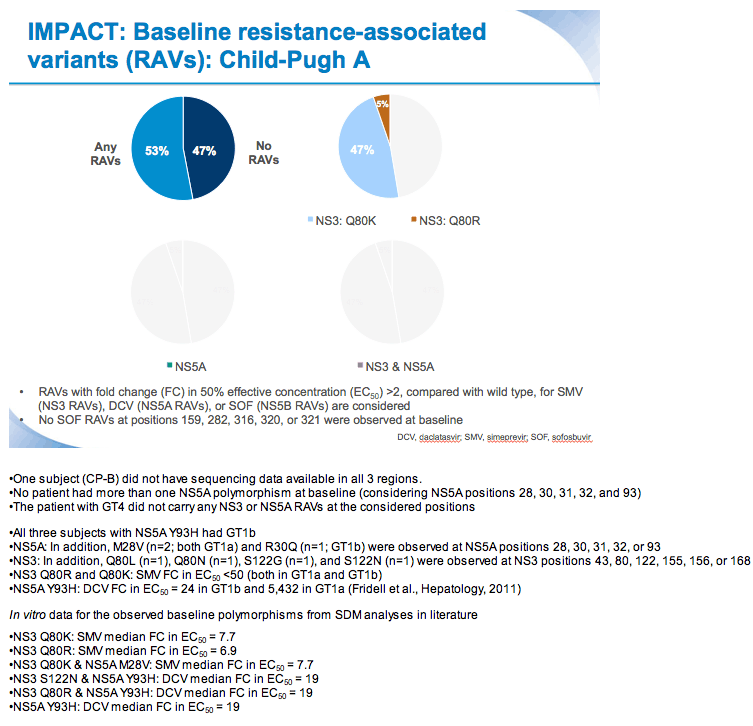
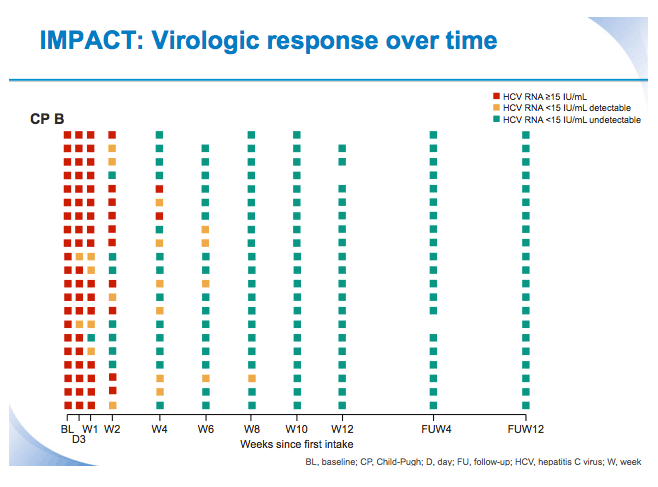
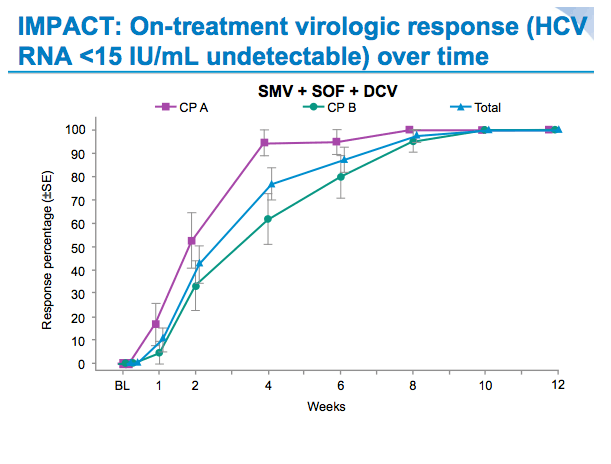
SVR12 results from the Phase II, open-label IMPACT study of simeprevir (SMV) in combination with daclatasvir (DCV) and sofosbuvir (SOF) in treatment-na´ve and -experienced patients with chronic HCV genotype 1/4 infection and decompensated liver disease
|
| |
| |
Reported by Jules Levin
AASLD 2015 Nov 13-17 San Francisco, CA
Eric Lawitz,1 Fred Poordad,1 Julio Gutierrez,1 Thomas N Kakuda,2 Gaston Picchio,3
Greet Beets,4 Ann Vandevoorde,4 Pieter Van Remoortere,2 Bert Jacquemyn,4
Gemma Quinn,4 Donghan Luo,2 Sivi Ouwerkerk-Mahadevan,5 Leen Vijgen,4
Veerle Van Eygen,4 Maria Beumont5
1Texas Liver Institute, University of Texas Health Science Center, San Antonio, TX, USA;
2Janssen Research & Development LLC, Titusville, NJ, USA; 3Janssen Research & Development, San Diego, CA, USA; 4Janssen Infectious Diseases BVBA, Beerse, Belgium;
5Janssen Research & Development, Beerse, Belgium
|
| |
|
 |
 |
|
|