 |
 |
 |
| |
Summary from AASLD 2015 for Hepatitis C
Beyond 95% SVR cure rates: still room for improvement?
|
| |
| |
Jürgen K. Rockstroh M.D., Professor of Medicine
University of Bonn, Germany
Correspondence:
Prof. Dr. J.K. Rockstroh
Department of Medicine I
University of Bonn
Sigmund-Freud-Str. 25
53105 Bonn
Germany
Introduction
HCV SVR rates of modern all oral DAA combination therapy are on average above 95% at least for genotypes 1 and 4, raising the question whether there is still room for new HCV drugs to be developed. Most interestingly, at this year AASLD a whole series of various dual and triple HCV DAA combinations were presented, some in earlier, some in quite advanced stages of clinical development, which promise broader cross-genotype activity including genotype 3, a potentially higher barrier to resistance, and in some instances maybe even shorter treatment durations. In light of the need to further simplify HCV diagnostics if HCV therapy is to be rolled-out globally (including resource limited settings), the prospect of all oral HCV therapies which may work for all patients regardless of HCV genotype or fibrosis stage is really exciting. In addition to future drugs a wealth of real life HCV treatment data was presented, which not only underlines the high efficacy and great safety of these new regimens, even in more challenging to treat patient populations but allows re-discussing patient groups which may be successfully treated with shorter treatment durations. Finally more clinical trial data on how best to treat genotype 3 as well as cirrhotics and patients with renal insufficiency was presented. Other special patient populations for which clinical trial data was presented for the first time was a whole series of studies looking at DAA therapy in patients with acute hepatitis C and then a study looking at efficacy and safety of all oral DAA combination therapy in patients who inject drugs while being on opioid agonist therapy. First results indicate that shortening of treatment duration for therapy of acute HCV with sofosbuvir and ribavirin may not work particularly in the setting of higher viral loads but that DAA combination therapy may be more promising. The study in patients with ongoing injection drug use which obtained once again very high SVR cure rates suggests we should not exclude these patients from HCV care but also reminds us all that successful HCV therapy is only one part of a successful management plan in these individuals but that we also need to maintain and improve prevention measures as reinfection rates in this study were high. The following review aims at summarizing the findings from the main HCV studies presented at AASLD. However, due to the very high number of papers being presented this overview is restricted to the personally conceived highlights and studies with potential clinical impact.
Use of DAA-based therapy for treatment of acute HCV
Up to now no data from all oral DAA therapy for treatment of acute HCV is available, thus most guidelines still recommend pegylated interferon +/- ribavirin for treatment of acute HCV infection. At this year AASLD meeting two studies and one case series were presented which examined the efficacy and safety of sofosbuvir + ribavirin combination for treatment of acute HCV in pilot trials (1-3). The first study was the ACTG study (A5327) also named as SWIFT-C which was an open label, two-cohort clinical trial (N=44). The first cohort received sofosbuvir 400mg QD + weight based ribavirin for 12 weeks with complete results presented here at this meeting (N=17) (1). The second cohort is currently being treated with ledipasvir/sofosbuvir (LDV/SOF) FDC QD for 8 weeks and is currently still enrolling (N=27). Main inclusion criteria were acute HCV infection (<24 weeks), or re-infection after clearance. Acute HCV was defined as new detectable HCV RNA and ALT ≥ 5XULN (>250 U/L) if normal ALT <12 mo or ALT≥ 10XULN (>500 U/L) and no ALT baseline or negative HCV Ab or RNA in prior 6 months. It needs to be pointed out that enrollment took place ≥12 and <24 weeks from first laboratory evidence of acute infection to allow time for spontaneous clearance. The baseline characteristics of the study population within cohort 1 are depicted below in Table 1.
Table 1: Demographics and baseline characteristics of cohort 1
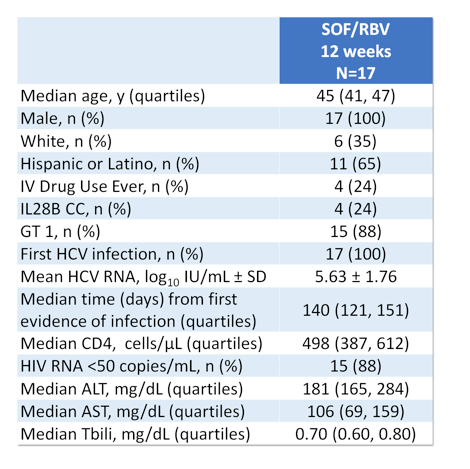
The relatively long median time from first evidence of infection (over 4 months) as well as the quite high HCV-RNA are noteworthy as they may have impacted the overall SVR rates. Figure 1 summarizes the main findings from the study.
Figure 1: SVR12 rates from the C-SWIFT study
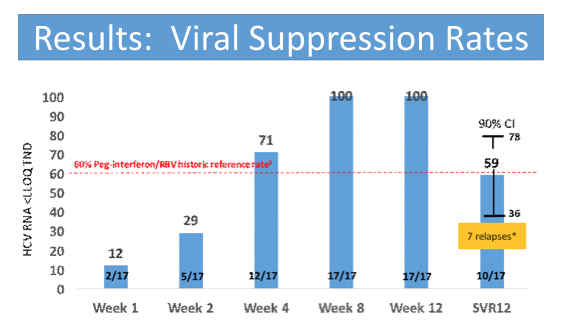
Overall, the observed SVR12 rate of only 59% is very disappointing and suggests that shorter treatment durations with sofosbuvir and ribavirin are not going to be the way forward in treating acute HCV infection.
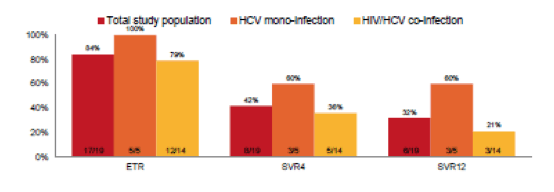
Indeed an even shorter treatment duration of only 6 weeks of sofosbuvir and ribavirin was studied in the Australian DARE study (2). In this study 19 patients were included. Acute HCV was defined by first positive anti-HCV antibody (Ab) and/or detectable HCV RNA within 6 months of enrolment and either acute clinical hepatitis within the past 12 months (symptomatic seroconversion illness or ALT greater than 10 times the upper limit of normal [ULN]) or documented anti-HCV Ab seroconversion within 18 months. Mean age was 42 years, 89% of study participants were male (n=17), 74% were HIV positive, 68% had GT1a (n=13) and 26% had GT3a infection (n=5). The principal modes of acquisition were injecting drug use (n=10, 53%) and sexual exposure with a partner of the same sex (n=8, 42%). Median HCV RNA at screening was 5.7 log10 IU/mL (IQR 5.0-6.3). In this study even lower SVR rates were noted again suggesting that the combination of sofosbuvir and ribavirin for shortned treatment durations may not be the ideal treatment of acute HCV. Noteworthy is the somewhat more liberal definition of acute HCV which may lead to the inclusion of early chronic HCV infected patients which may also lower overall SVR rates. The end-of-treatment results (ETR), SVR4 and SVR12 rates for HCV mono, HIV/HCV coinfected and the entire study poulation are depicted in figure 2.
AASLD: Sofosbuvir Plus Ribavirin Without Interferon for Treatment of Acute HCV in HIV-1 Infected Individuals: SWIFT-C....Harvoni 8 weeks enrolling - (12/04/15)
Figure 2: Primary and secondary efficacy endpoints - overall and by HIV status.
These rather disappointing findings were contrasted by the report of a patient series from New York where treatment of acute HCV with sofosbuvir and ribavirin for 12 weeks was associated with a SVR12 rate of 92% (11/12) (3). Indeed only 1 of 12 treated patients developed a relapse. These patients were all not included into the ACTG study reported above because of not fulfilling an inclusion criteria or presenting with exclusion criteria to the study. The definition of acute HCV was: any new ALT elevation, with other causes excluded, in the setting of HCV viremia and Ab seroconversion for primary infection; and for re-infection, additional documentation of not-detected VL for the prior ≥12 weeks. There was a 12-week observation period for spontaneous clearance before treatment initiation. Interestingly the initiation of HCV treatment occurred after a median duration of 22 weeks (IQR 15, 34) after first detection of acute HCV, which was even a little bit longer than in the ACTG study. The long time before starting therapy was partially related to obtaining cost approval from health insurance companies before commencing HCV therapy. Seven of the patients were white, 2 Hispanic, 2 black, and 1 Asian; 7 had CC and 5 had CT IL28B polymorphisms; 10 had genotype 1a and 2 had genotype 1b HCV. Median treatment-baseline VL was 4.47 log10 IU/mL, although two had very high VL of >7 log10 IU/mL. Indeed the median HCV viral load was considerably lower than in the ACTG study and may explain partially the observed differences in response.
AASLD: SOFOSBUVIR IN THE TREATMENT OF ACUTE HCV INFECTION IN HIV-INFECTED MEN - (12/04/15)
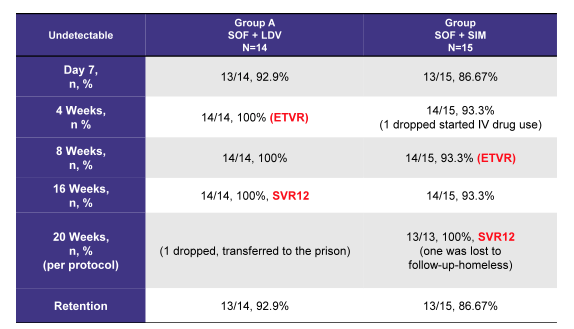
Finally, first data from a pilot trial on treatment of acute HCV with sofosbuvir and ledipasvir versus sofosbuvir and simeprevir combination was presented in the poster session (4). Overall, 29 patients with a diagnosis of acute hepatitis C (defined as negative past HCV antibody + new onset HCV RNA) were recruited from 6 inner city drug rehab programs in Brooklyn, NY. No information was provided on how long the potential observation period from last negative HCV Ab was. Patients included into the pilot trial were divided into 2 groups; Group A (n=14): SOF 400 mg + LDV 90 mg (once daily) - 4 weeks Group B (n=15): SOF 400 mg + SIM 150 mg (once daily). All patients had a genotype 1 infection (7/7 GT1a/1b in group A and 7/8 GT1a/GT1b in group B, respectively). Mean viral load was 1,200 k in the SOF + LDV group and 1,600 k in the SOF + SIM group (unfortunately no SD was provided). Overall, very high SVR rates above 90% were achieved following very short all oral DAA combination therapy suggesting that indeed DAA combinations may be more efficacious than shortened durations of sofosbuvir and ribavirin. The somewhat lower mean HCV viral load than in the sofosbuvir + ribavirin trials may be one reason for the improved response rate. To better interpret the results standard deviation as well as a more clear definition of what was considered acute HCV would be needed. The ETR and SVR 12 data are shown below in table 2. Clearly, DAA combinations need to be explored in more trials in order to obtain more clear guidance on how and who to treat during acute HCV.
Table 2: SOF/LDV vs. SOF + SIM for Acute Hepatitis C: Results
AASLD: Sofosbuvir and Ledipasvir versus Sofosbuvir and Simeprevir combination therapy in the management of acute hepatitis C: A randomized open label prospective clinical pilot study. SLAM C study - (12/04/15)
Feedback from real-life cohorts: what can we learn?
Different treatment durations possible?
Real-life cohorts offer the advantage of evaluating new therapies in somewhat more challenging patient populations, which may not be included into clinical trials. For example these cohorts on average have a much larger subgroup of patients above 65y of age; usually more advanced liver disease stages and potentially also more adherence-challenged patient groups. In particular the recommendation which derived from clinical trials to treat patients with GT1 infection who were treatment naïve and had no cirrhosis with only 8 weeks of ledipasvir/sofosbuvir (LDV/SOF) if HCV viral load was below 6 Mill IU/ml has been challenged. At this year AASLD 8 vs 12 weeks of LDV/SOF were evaluated in multiple cohorts. One of the cohorts to present data on this issue was through Trio Health's Innervation Platform, a cloud-based disease management platform, and directly from specialty pharmacies for 895 treatment-naïve patients with non-cirrhotic GT1 Hepatitis C who initiated 8 or 12 weeks LDV/SOF between Oct 2014 and 2015 (5). Table 3 summarizes the main HCV relevant baseline disease characteristics. Clearly patients who were treated for only 8 weeks were less likely to have advanced fibrosis and on average had much lower HCV viral loads.
Table 3: Baseline characteristics from the TRIO network
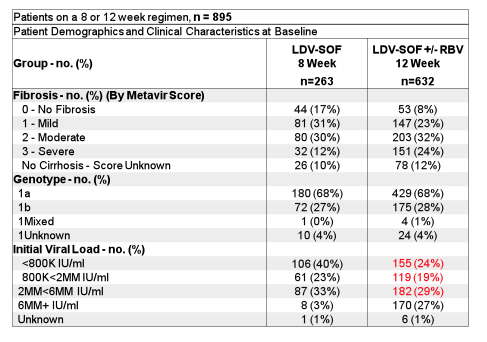
An amazingly high SVR12 rate was observed for both treatment durations: 95% (251/263) for the 8 week treated patients and 96% (604/632) for the 12 week treated patients, respectively. SVR12 rates by baseline fibrosis stage and HCV viral load above or below 6 Mill IU/ml are shown in Figure 3a+b.
Figure 3a+b: Effectiveness of 8 or 12 week LDV/SOF in GT1, treatment-naïve, non-cirrhotic HCV patients
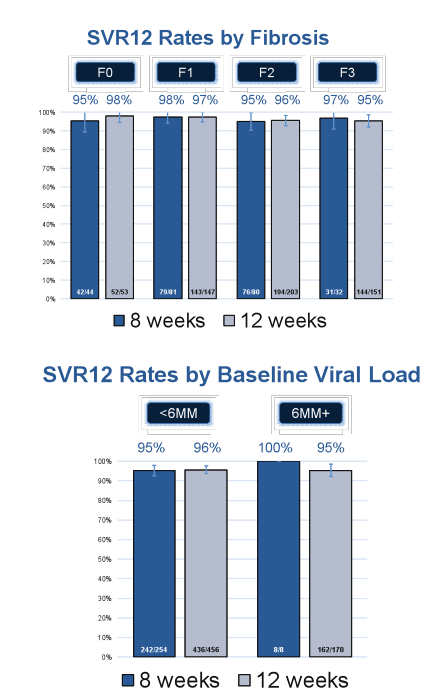
Interestingly, 12 week therapy was selected by physicians for 456 patients with an HCV- RNA < 6MM IU (51%), indicating physician preference for longer therapy. Overall, this could imply that potentially physicians felt more comfortable only treating their "easiest to treat patients" with the shorter duration. Nevertheless the high and comparable SVR12 rates suggest that shorter treatment durations may need to be discussed particularly under cost saving considerations.
AASLD: Effectiveness of 8 or 12 week LDV-SOF in Treatment-Naïve Patients with Non-Cirrhotic, Genotype 1 Hepatitis C: Real-World Experience from the TRIO Network - (12/16/15)
Interesting data on 8 weeks of therapy with ledipasvir/sofosbuvir was also presented from Germany from the GECCO cohort (6). The GECCO cohort is a multicenter cohort from 8 sites in Germany. All patients started on the following DAAs were included in the analysis: Sofosbuvir (SOF), pegylated interferon and ribavirin (RBV); SOF and RBV; SOF and simeprevir (SMV); SOF and daclatasvir (DCV) +/- RBV; SOF and ledipasvir (LDV); paritaprevir/ritonavir (PTV/r), ombitasvir (OBV)+/- RBV and +/- dasabuvir (DSV). All GECCO patients are part of the German hepatitis C registry. This particular sub analyses only reports on patients with GT1 and 4 treated with SOF and LDV for 8 weeks (SL8). Overall, the majority of patients were male (n=72; 49%) and the median age was 52 years. 144 patients (97%) were GT1 infected, n=3 GT4 infected. 28 (19%) were HIV-HCV coinfected with a median CD4 cell count of 531/mm3 (346-683). N=5 (3%) had F4 Fibrosis defined as Fibroscan > 12,5 KPa or APRI > 2. Baseline HCV viral load was measured with either Roche Taqman v2.0 (used by 4 sites, n=79) or Abbott Real Time PCR (used by 3 sites, n=54, one site used both). N=13 (8,8%) had a high viral load (HVL) defined as > 6 Mill. IU/mL (Roche) or > 2 Mill. IU/mL (Abbott) at baseline. Interestingly, there was a subgroup of patients which was treated for 8 weeks with ledipasvir/sofosbuvir beyond the label recommended criteria: baseline HCV viral load above 6 Mill IU/ml, F4-fibrosis, prior HCV therapy and genotype 4. SVR 4 and 12 rates (SL8) are depicted in figure 4 in different subgroups (ITT).
Figure 4a+b: SVR 4 rates (SL8) in different subgroups (ITT) (4a) and SVR 12 rates (SL8) in different subgroups (ITT) (4b)
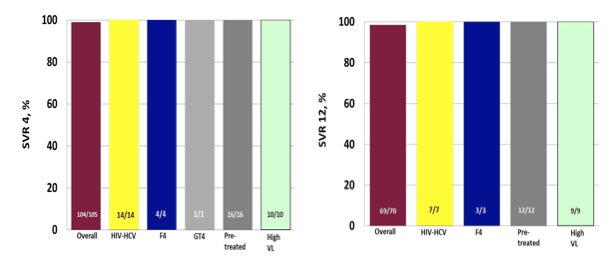
Sofosbuvir plus ledipasvir for 8 weeks (SL8) achieved excellent response rates in a German real-life setting. HIV coinfected patients responded as well as HCV monoinfected patients. High SVR rates were even seen in patients treated, outside recommendation (HVL, F4, prior treatment, GT4). SL8 was safe and well tolerated. Clearly the 8 week treatment regimen needs to be revaluated particularly when more and more patients are going to be treated which do not already have advanced fibrosis stages.
Finally, the safety and efficacy of LDV/SOF containing regimens for the treatment of patients with chronic hepatitis C GT 1 infection was also evaluated in HCV-TARGET, a multicenter, prospective, observational cohort study (7). Patients who initiated HCV treatment in clinical practice were enrolled and treated according to the local standards of care at academic (n=38) and community medical centers (n=13) in North America (n=47) and Europe (n=4). Information was collected from the medical records and abstracted into a unique centralized data core. Independent data monitors systematically reviewed data entries for completeness and accuracy. Demographic, clinical, adverse events (AEs) and virological data were collected throughout treatment and post-treatment follow-up. The AASLD presentation from this year aimed at comparing 8 versus 12 weeks of LDV/SOF therapy as well as impact of baseline factors on treatment outcome in this real-word cohort of chronic HCV GT1 patients from TARGET. Overall, 1270 patients who completed HCV therapy prior to 1st July 2015 were included into the analysis. The status of treated GT1 patients within TARGET is depicted in figure 5.
Figure 5: HCV-TARGET: Status of LDV-SOF Treated G1 Patients
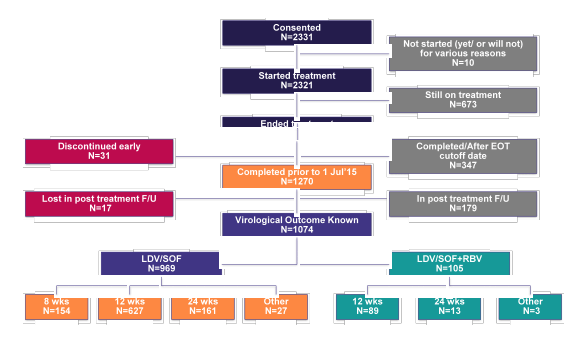
Overall, of the 1270 included patients 1139 patients received LDV/SOF and 131 LDV/SOF + RBV. Patients who received additional ribavirin were much more likely to be cirrhotic and have a history of prior treatment failure (35 vs 63% and 44 vs 69%, respectively). Patients which were treated for 8 weeks only (n=154) were much more likely to be treatment-naïve and have no cirrhosis. The corresponding SVR rates for the different treatment durations and schedules (with or without RBV) are summarized in figure 6.
Figure 6: HCV-TARGET: SVR12 and Relapse Rates
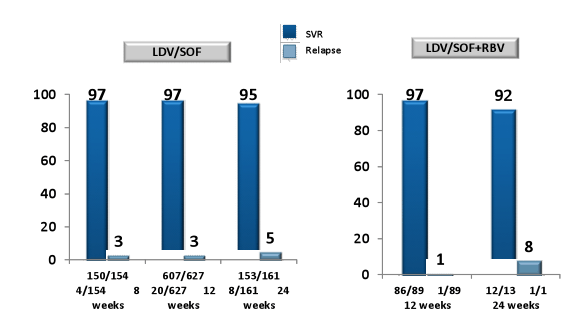
In summary, preliminary safety and efficacy data from HCV-TARGET suggests that LDV/SOF-containing 8, 12 and 24-wk treatment regimens are generally safe, well tolerated, and highly effective across a broad spectrum of patients and clinical practices. Notably, fibrosis stage and treatment history determined whether ribavirin was added as well as treatment duration. But even if the 8 week treated patients were easier to treat it remains fair to say that those less difficult to treat individuals may get away with 8 weeks of therapy which could lead to considerable cost savings. Reassuringly, the more advanced patients which more often received the LDV/SOF + RBV therapy or 24 weeks of therapy also had very high HCV cure rates very much in the range what has been reported from clinical trials.
In a next analysis the authors attempted to evaluate impact of baseline factors on treatment outcome. Interestingly, use of proton pump inhibitors (PPI) was also examined as one possible baseline factor. Inverse probably weighting (IPW) was applied to make PPI and non-PPI groups comparable on other baseline factors. The LASSO method was used to define candidate predictor variables. The findings of the multivariable logistic model for SVR with LDV/SOF are shown in figure 7.
Figure 7: HCV-TARGET: Multivariable Logistic Model for SVR with LDV/SOF
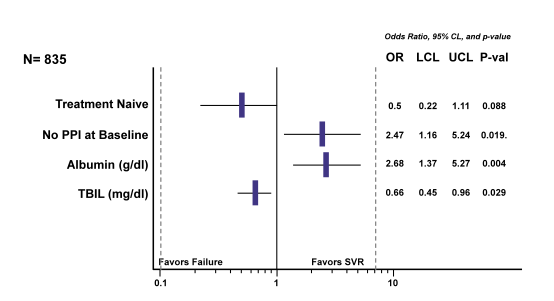
Most interestingly, use of PPIs was associated with a higher risk of virological failure. Clearly, this could be a potentially modifiable factor, which may help to further increase SVR rates. Whether the negative impact of PPIs derives from drug interactions or changes in drug absorption remains unclear at this time point.
AASLD: (HCV-TARGET) - Treatment Outcomes With 8, 12 and 24 Week Regimens of Ledipasvir/Sofosbuvir for the Treatment of Hepatitis C Infection: Analysis of a Multicenter Prospective, Observational Study - (12/03/15)
Efficacy and safety of all oral DAA combination therapy in real-world cohorts: any difference to clinical trials?
The other main aim of real-life cohorts was to assess the characteristics of patients with genotype 1 HCV who failed all oral DAA therapy. Within the TRIO network (see cohort description above) patients who received 12 weeks of LDV/SOF, VKP or other all-oral DAA therapies and developed virological failure were evaluated (8). All genotype 1 HCV patients who initiated treatment with 12 week LDV/SOF, VKP or simeprevir + sofosbuvir (SMV+SOF)-based regimens between Oct 2014 and 2015 were included in the analysis (n = 1685). The patient disposition is summarized below in figure 8, the SVR12 rates for the different regimens in figure 9, respectively.
Figure 8: Real-world experience from the TRIO Network: Patient distribution and SVR rates with all-oral DAA regimens
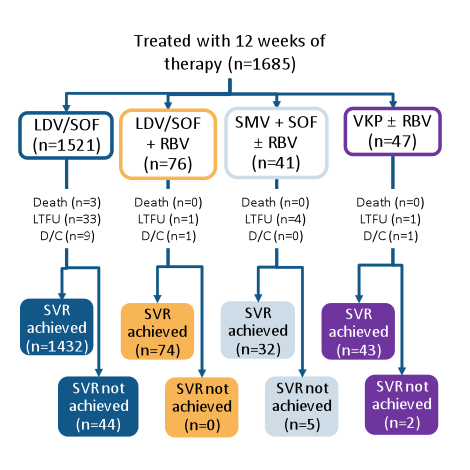
Figure 9: SVR12 rates by regimen in the TRIO network
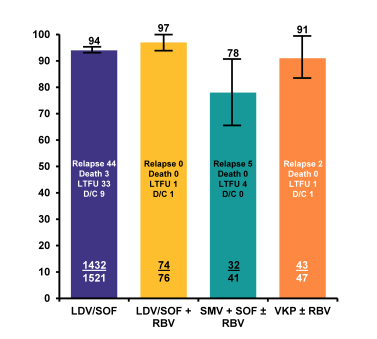
Overall SVR in real world HCV genotype 1 patients was extremely high and between 78 and 97%. Treatment discontinuation was not a major issue with any all oral DAA therapy combination underlining the increased safety and tolerability of modern all oral DAA combination therapy. Further sub analyses looked at potential predictors for treatment outcome was able to identify low platelets, presence of cirrhosis, male gender and prescribing HCV therapy outside FDA guidelines (Patients outside of guidelines: GT1a on VKP without RBV, tx failure cirrhotic patients on 12 weeks of VKP+/-RBV, LDV-SOF without RBV, or SMV+SOF+/-RBV) as predictors for treatment failure (see table 4).
Table 4: TRIO results: predictors of HCV treatment response
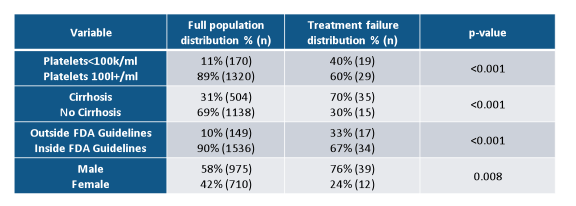
In summary, overall SVR in real world genotype 1 HCV patients was above 95% across regimens and patient characteristics and thereby almost superimposable to treatment outcome observed in randomized clinical trials. Cirrhosis, thrombocytopenia and prior treatment failure represented the most difficult to treat patients. Treatment discontinuation was not a major issue with any all oral DAA therapy.
Interesting efficacy and safety data was also presented for other genotypes. Tanja Welzel reported back on safety and efficacy of sofosbuvir containing regimens for GT2 and GT3 HCV-TARGET participants (9). This analysis included all patients who started SOF/RBV before 10/10/14 (GT2) or 7/15/14 (GT3) and for whom a known outcome was available. 23% of the 256 GT2 patients who were treated with sofosbuvir and ribavirin for 12 week were cirrhotic at baseline. More patients were cirrhotic in the 16 week treatment group (56% of 39). In the GT3 patients 55% of 130 subjects had cirrhosis at baseline; all patients received 24 weeks of sofosbuvir plus ribavirin therapy. Figure 10 shows the main SVR12 findings for the different genotypes and patient characteristics. From these findings it becomes very evident that sofosbuvir + ribavirin is far from ideal for treatment of patients with cirrhosis and prior treatment failure. This is true to a lesser extent for genotype 2 but really dramatically for genotype 3 with less than 50% SVR12 rate in cirrhotic previous treatment failures again highlighting that development of new compounds will be crucial for improving treatment success rates in this special patient population.
Figure 10: Unadjusted SVR12 rates with SOF/R from the TARGET cohort
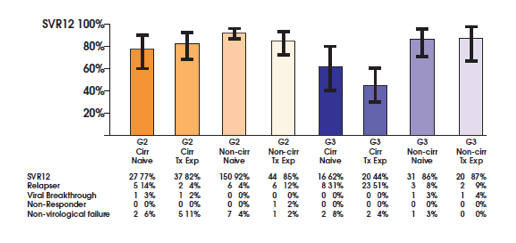
Anything new for genotype 3 with currently licensed agents?
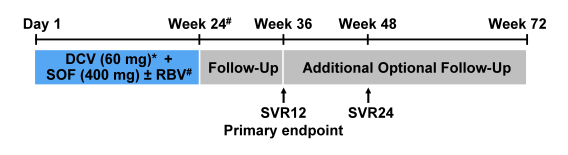
The ALLY-3 study showed great SVR12 rates of 96% after 12 weeks of sofosbuvir + daclatasvir in treatment naïve patients without cirrhosis. In patients with cirrhosis however SVR rates were substantially lower with only 63% (10). Therefore new treatment strategies for genotype 3 infection in patients with more advanced liver disease are urgently needed. At this year AASLD the results of the ALLY-3+ study were presented which investigated daclatasvir (DCV) + sofosbuvir (SOF) + ribavirin (RBV) for 12 or 16 weeks in GT 3-infected patients with advanced fibrosis (F3) or compensated cirrhosis (F4) (11). The study design is shown in figure 11.
Figure 11: Ally-3+ Study design: Phase 3b, open-label, randomized study
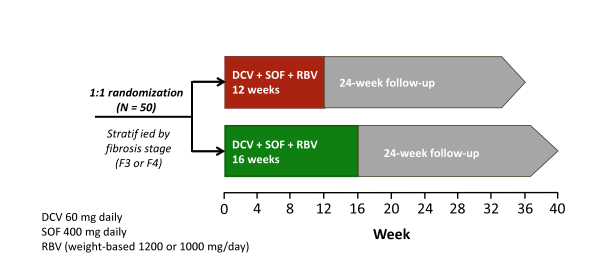
The Ally-3+ study allowed evaluating whether the addition of ribavirin would help to improve SVR rates in these more challenging to treat patients. Unfortunately though patient number included was small (only 50 subjects) and also the question whether perhaps extending therapy to 24 weeks plus adding ribavirin is helpful or not cannot be answered. The table 5 below captures the main baseline characteristics of the study population examined. Three quarters of patients had cirrhosis and half a HCV viral load above 6 Mill IU/ml suggesting that this really was a more difficult to treat patient population.
Table 5: Baseline characteristics of the Ally-3+ study population
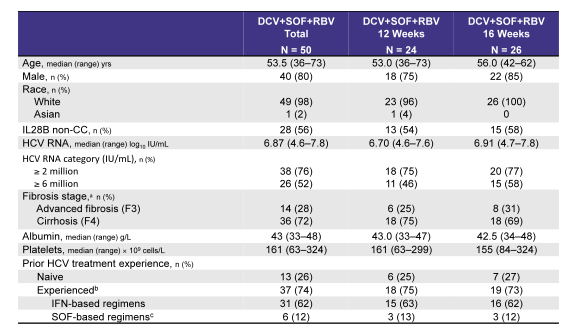
Figure 12 shows the overall HCV cure rates by prior treatment and in cirrhotics. Overall, SVR rates are much high than without ribavirin in the ALLY-3 trial for GT3 patients with cirrhosis but still cure rates are below 90% and therefore the question remains whether further extension of treatment duration could improve SVR rates or not. Clearly addition of 4 weeks was not able to further improve SVR rates over 12 weeks of combination therapy plus ribavirin.
Figure 12: SVR12 rates by prior treatment history
Various studies were presented on the combination of sofosbuvir and daclatasvir +/- ribavirin from compassionate use programs. Although these studies are important, as they give a feedback from the most difficult to treat patient populations with regard to the efficacy and safety of this particular regimen, in the absence of randomization and more advanced patients tending to receive additional ribavirin, no answer can be provided on which patient type may require additional ribavirin or not. The study design of the European compassionate use program (CUP) is shown below in figure 13 (12). This CUP enrolled adult patients with chronic HCV infection who were at a high risk of hepatic decompensation or death within 12 months if left untreated, and who had no available treatment options. Patients received DCV 60mg + SOF 400mg QD, with RBV added at the physician's discretion. Recommended duration of treatment was 24 weeks.
Figure 13: Study design of the CUP study
The baseline characteristics of the study population are shown in figure 14 and highlight the advanced liver disease stages of the included study patient population.
Figure 14: Baseline characteristics of the CUP program
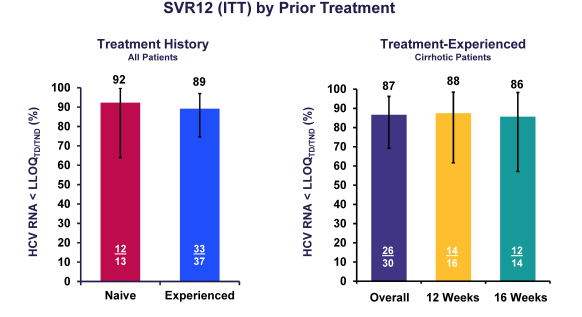
Indeed 85% of patients had cirrhosis, and 59% were prior treatment failures. Median platelets were low with 75 x109/L. Considering the enrichment of unfavorable predictors for response in this patient population the overall SVR rates are quite impressive. Indeed overall 87% of patients achieved SVR12. Please see treatment outcome results summarized in figure 14.
Figure 15: Primary efficacy analysis - SVR12 (mITT) in GT3 patients
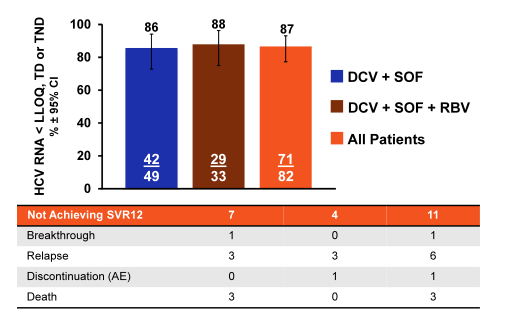
In conclusion, in a real-life clinical setting, DCV + SOF ± RBV achieved high SVR rates (86%) in HCV GT 3-infected patients at high risk of hepatic decompensation or death with 87% SVR12 in patients with confirmed cirrhosis (including decompensated cirrhosis). Comparable SVR12 rates were obtained with or without RBV in this regimen, but again this study was not designed to evaluate the role of ribavirin in this particular patient group.
AASLD: All-Oral Treatment With Daclatasvir Plus Sofosbuvir Plus Ribavirin for 12 or 16 Weeks in HCV Genotype 3-Infected Patients With Advanced Fibrosis or Cirrhosis: The ALLY-3+ Phase 3 Study - (11/23/15)
AASLD: Safety and Efficacy of Daclatasvir Plus Sofosbuvir With or Without Ribavirin for the Treatment of Chronic HCV Genotype 3 Infection: Interim Results of a Multicenter European Compassionate Use Program - (11/16/15)
AASLD: Daclatasvir Plus Sofosbuvir With or Without Ribavirin for the Treatment of Chronic HCV in Patients Coinfected With HIV: Interim Results of a Multicenter European Compassionate Use Program - (11/18/15)
Anything new in special patient populations?
HIV/HCV coinfection
In the ALLY-2 study which in two of the study arms evaluated 8 versus 12 weeks of daclatasvir (DCV) and sofosbuvir (SOF) in treatment naïve HIV/HCV coinfected individuals found significantly lower SVR12 rates after 8 weeks vs 12 weeks of combination therapy (75.6% vs 96.4%, respectively) (13). One of the proposed potential reasons for this underperformance was insufficient daclatasvir drug levels. At the time when ALLY-2 was conducted all patients on a boosted PI only received half the daclatasvir dose (30mg) as increases of daclatasvir were expected based on drug-drug interaction studies. DCV dose reduction in ALLY-2 with DRV/r and LPV/r was based on extrapolation of observed pharmacokinetic (PK) DDI data showing approximately 2-fold increase in steady-state DCV systemic exposure after coadministration with ATV/r (Study AI444-032). In parallel to ALLY-2, however a dedicated DDI study (AI444-093) showed substantially smaller increases in DCV AUC with concomitant DRV/r (1.41-fold) or LPV/r (1.15-fold) than had previously been determined for ATV/r (2.1-fold) (14). Based on the results of this study, the standard DCV 60 mg dose is now recommended during coadministration with DRV/r and LPV/r. This implies that potentially the patients in ALLY-2 who were on DRV/r may have been underdosed with daclatasvir. Now at AASLD the results of the ALLY-2 PK Analysis were presented which was conducted to clarify whether lower daclatasvir exposure in darunavir treated patients may account for the higher failure rate in the 8 week treatment arm (15). Plasma samples to determine DCV PK were collected pre-dose from all patients during treatment weeks 1, 2, 4 (includes sampling at predose, 0.5 and 4 hrs post-dose), 8, and 12 Composite DCV trough plasma concentrations (Ctrough) were derived for all patients from individual DCV Ctrough concentrations determined by validated LC-MS/MS methodology. Ctrough concentrations in DRV/r or LPV/r recipients were stratified by SVR12 and treatment duration and observed composite Ctrough was compared visually between patients who did or did not receive DRV/r or LPV/r, and between patients who did or did not achieve SVR12. Figure 16 shows the PK data for the composite DCV Ctrough by SVR12 and DCV Dose.
Figure 16: ALLY-2: Composite DCV Ctrough by SVR12 and DCV Dosea
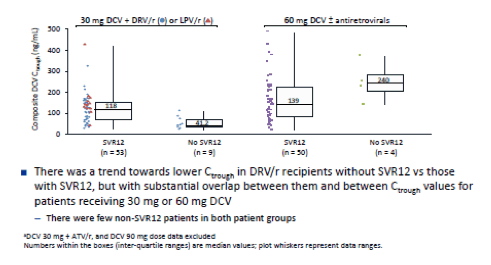
In conclusion, although dosing of DCV 30 mg in HIV-HCV coinfected patients receiving DRV/r may have contributed to cases of virologic failure, the data suggest that low DCV systemic exposure was not the primary cause of relapse in ALLY-2 patients receiving DCV 30 mg with DRV/r. Relapse among patients receiving DRV/r with 30 mg DCV in ALLY-2 may be associated primarily with shorter (8-week) treatment duration and high baseline HCV RNA.
AASLD: Daclatasvir Exposure Alone Does Not Explain HCV Relapse in HIV-HCV Coinfected Patients Receiving Daclatasvir Plus Sofosbuvir with Ritonavir-Boosted Darunavir in the ALLY-2 Study - (11/18/15)
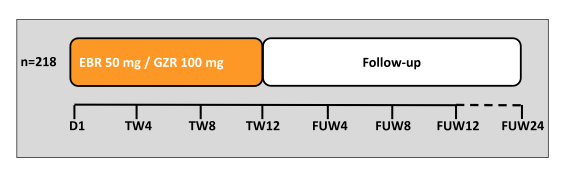
Another interesting presentation in the area of HIV/HCV coinfection was the presentation of the final SVR24 results from the C-EDGE coinfection study which examined virological efficacy and safety of the fix-dose combination of grazoprevir/elbasvir for 12 weeks in 218 treatment-naive patients with HCV GT1, 4 or 6 infection with or without cirrhosis (16,17). Patients were co-infected with HIV-1 and either naive to ART with CD4+ >500 cells/mm3 and HIV RNA <50,000 copies/mL or on stable on ART for ≥8 weeks and CD4+ >200 cells/mm3 and undetectable HIV RNA. Stable antiretroviral therapy (ART) included tenofovir or abacavir, and either emtricitabine or lamivudine plus raltegravir, dolutegravir, or rilpivirine. The study design is shown in figure 17.
Figure 17: Study design of the C-EDGE coinfection study
Overall, 83% of study participants were male, 17.4% were black or African-American, and 66.1% had a GT1a, 20.7% GT1b /other, 12.8% GT4 and 0.5% GT6 HCV infection. 16.1% were cirrhotic, 35.3% had an IL28B CC genotype and 59.6% had a baseline HCV RNA >800,000 IU/mL. 96.8% were receiving ART with undetectable HIV RNA. The median (1st quartile - 3rd quartile) CD4 count was 568 cells/μl (424-766). 21.6% of patients were on an abacavir containing ART regimen, 75.2% on a tenofovir containing regimen; 51.8% received raltegravir as third agent, 27.1% dolutegravir and 17.4% rilpivirine. The results of the secondary study endpoint (SVR24 = HCV RNA <15 IU/mL) is depicted below in figure 18.
Figure 18: SVR24 results: Full analysis set (primary analysis which includes all patients and reinfections are counted as failure
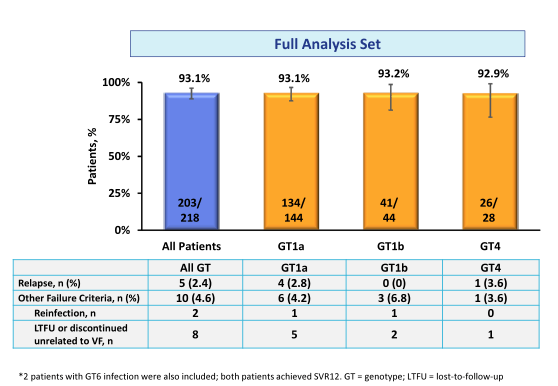
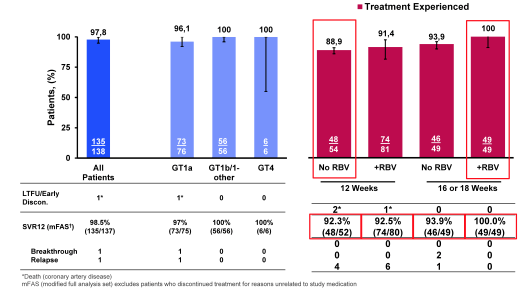
Interestingly 2 patients developed reinfection with a different genotype, which were counted as failure in the primary analysis reminding us that treating with DAAs alone is not enough but that we need to actively counsel patients about risk of becoming infected again. In the modified full analysis set (post-hoc supportive sensitivity analysis which excluded patients who discontinued the trial for non-treatment related reasons (e.g. lost-to-follow-up and non-virologic failure) as well as excluded reinfections) overall SVR24 was very high with 97.6%. No relapse was noted in GT1b patients underlining the high efficacy of this regimen in GT1b patients. Overall safety was very good. No treatment discontinuation because of treatment related adverse events was noted. In summary, high rates of SVR were achieved in patients with HCV GT1, 4 and 6 and HIV coinfection receiving the all-oral, fixed-dose combination of EBR/GZR. The HCV cure rate was comparable to the response rates to other EBR/GZR studies in HCV mono-infected patients such as the SVR12 of 95% in C-EDGE treatment naïve patients (18).
AASLD: C-EDGE Co-Infected: final results from Phase 3 Study of elbasvir / grazoprevir in Patients with HCV/HIV - (11/30/15)
THE PHASE 3 C-EDGE TREATMENT-NAIVE (TN) STUDY OF A 12-WEEK ORAL REGIMEN OF GRAZOPREVIR (GZR, MK-5172)/ELBASVIR (EBR, MK-8742) IN PATIENTS WITH CHRONIC HCV GENOTYPE (GT) 1, 4, OR 6 INFECTION - (04/24/15)
HCV-infected Persons Who Inject Drugs on Opioid Agonist Therapy
In general persons who inject drugs are often left behind in the linkage to care and management of hepatitis C mostly because of the perception that their compliance will be worse and outcome of therapy would be different. Therefore it was a great achievement to follow the presentation of the C-EDGE COSTAR study which is a Phase 3 trial evaluating efficacy and safety of GZR/EBR among treatment naïve HCV GT1/4/6-infected patients ± cirrhosis who are receiving opioid agonist therapy (OAT) (methadone or buprenorphine). The study design is shown below in figure 19.
Figure 19: Study design of the C-EDGE COSTAR study.
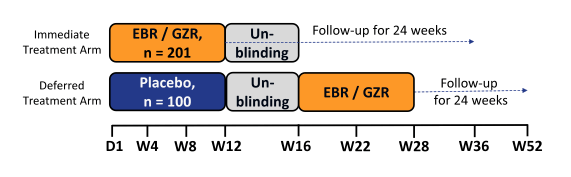
This double-blind, placebo-controlled study randomized patients 2:1 to an immediate treatment group (ITG) or a deferred treatment group (DTG). The ITG received GZR/EBR for 12 weeks; the DTG received placebo for 12 weeks, followed by 12 weeks of open label GZR/EBR. Use of non-prescribed drugs was monitored by urine drug screening, but was not exclusionary to study participation. 301 patients were randomized (mean age 47 years; 76% male; 12% black; 76% GT1a; 21% cirrhotic, 7% HIV). At baseline, 79% were receiving methadone; 21% were receiving buprenorphine. In the ITG, 99% (199/201) completed 12 weeks of treatment, despite 79% with drug use (amphetamines 16%, benzodiazepines 39%, cannabinoids 40%, cocaine 19%, and opioids 41%) during treatment. The SVR12 rates for the full analysis set are depicted below in figure 20.
Figure 20: SVR12 rates in the fill analysis set of the C-EDGE COSTAR study
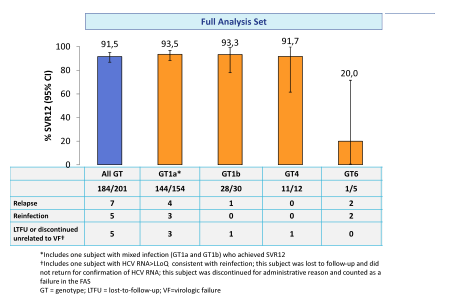
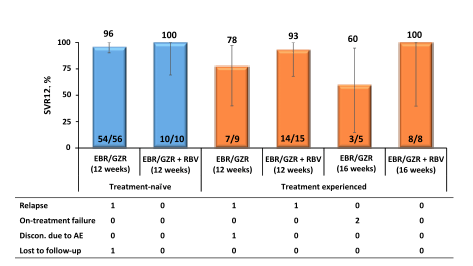
Overall good HCV cure rates were obtained for GT 1 and 4. Too few patients were included with genotype 6 to allow further conclusions. Noteworthy, 5 reinfections (confirmed by phylogenetic analyses) were recorded again emphasizing that prevention counseling as well as supply of clean needles and syringes is of utmost importance to prevent reinfection in this particular patient population. The high SVR rates also allow the conclusion that patients who do continue to inject drugs while on opioid agonist therapy do not need to be excluded from HCV therapy as their chance of SVR is comparable to that of other patient groups.
AASLD: C-EDGE CO-STAR: efficacy of grazoprevir / elbasvir Fixed Dose Combination for 12 Weeks in HCV-infected Persons Who Inject Drugs on Opioid Agonist Therapy - (11/16/15)
Any new data in patients with renal impairment?
An update of the RUBY study was presented which examined safety and efficacy of 12 weeks of the Abbvie 3-D regimen (Co-formulated ombitasvir and low dose ritonavir boosted paritaprevir (OBV/PTV/r) (25/150/100 mg QD) and dasabuvir (DSV) (250 mg BID) in non-cirrhotic HCV genotype 1 patients with chronic kidney disease with estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2 (20). This open-label phase 3b study was performed in the US at 9 different sites. Patients with genotype 1a received additional 200mg ribavirin (in patients on hemodialysis RBV was dosed 4 hours prior to start of hemodialysis) whereas patients with HCV genotype 1b were treated without ribavirin. Final results were reported with a SVR12 rate of 90%, which clearly is an excellent response rate for this challenging to treat population (see figure 21).
Figure 21: Ombitasvir-Paritaprevir-Ritonavir and Dasabuvir in GT1 & Renal Disease RUBY-I: SVR (ITT)
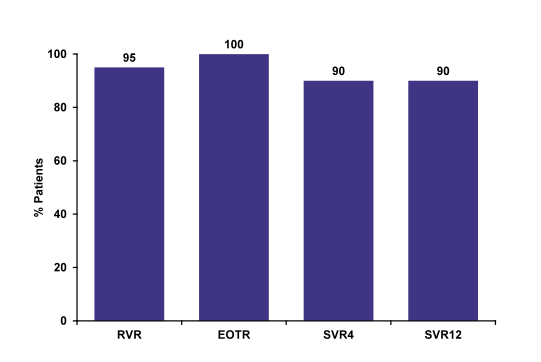
Among genotype 1 infected patients with stage 4 or 5 chronic kidney disease 3D+/-RBV has
been well tolerated, with no premature treatment discontinuations. Hemoglobin decreases were managed with RBV interruption, which does not appear to affect efficacy. Overall SVR12 rate is high with 90%.
AASLD: RUBY-I: Ombitasvir/Paritaprevir/Ritonavir + Dasabuvir ± Ribavirin in Non-Cirrhotic HCV Genotype 1-Infected Patients With Severe Renal Impairment or End-Stage Renal Disease - (12/16/15)
Any news for treatment of cirrhosis with the currently licensed agents?
Treatment with the 3 direct-acting antiviral (3D) regimen of ombitasvir, paritaprevir boosted with ritonavir, and dasabuvir without ribavirin (RBV) for 12 weeks has demonstrated 12-week sustained virologic response (SVR12) rates of 100% in HCV genotype (GT) 1b patients without cirrhosis, and 99% in GT1b patients with compensated cirrhosis when co-administered with RBV for 12 weeks. At AASLD the safety and efficacy of the 3D regimen without RBV in patients with HCV GT1b infection and compensated cirrhosis was reported from the TURQUOISE-III trial (21). Patients enrolled in this phase 3b, multicenter, open-label study received 12 weeks of 3D without RBV. Both treatment-naïve and peginterferon/RBV treatment-experienced patients with compensated cirrhosis with no history of decompensation were enrolled with the following criteria: hemoglobin ≥10 g/dL, albumin ≥2.8 g/dL, platelet count ≥25 x 109/L, and creatinine clearance ≥30 ml/min. The baseline demographics are shown below in figure 22.
Figure 22: Baseline characteristics for TURQUOISE-III: OBV/PTV/r + DSV in Genotype 1b Cirrhosis
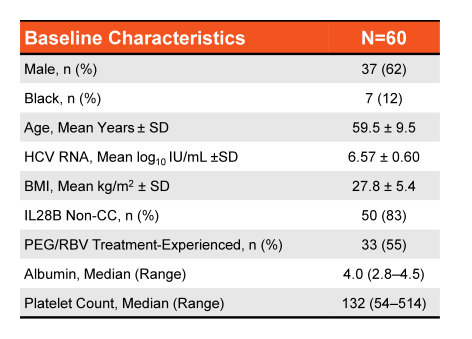
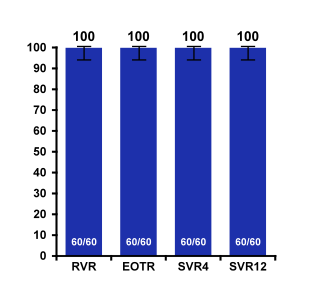
Median albumin and platelet count indicate that these are early stage cirrhosis which is important to remember also because there has been a recent FDA warning for using this HCV therapy in more advanced cirrhosis stages because of occurrence of severe liver injury. In these CHILD A patients however, no safety signals were noted and SVR12 was 100% emphasizing that this regimen requires no additional ribavirin in CHILDA cirrhotic patients with GT1b infection. The SVR outcomes are summarized in Figure 23.
Figure 23: SVR rates from the TURQUOISE-III trial: OBV/PTV/r + DSV in Genotype 1b Cirrhosis
AASLD: TURQUOISE-III: 12-Week Ribavirin-Free Regimen of Ombitasvir/Paritaprevir/r and Dasabuvir for Patients With HCV Genotype 1b and Cirrhosis - (12/22/15)
New HCV drug development
Clearly one of the main topics and points of recurrent discussion were the wealth of data around new HCV agents, which were presented often for the first time at this meeting. The new DAA combination closest to becoming approved as the next available HCV regimen is the fix-dose combination of elbasvir/grazoprevir (NS5A-inhibitor/HCV protease inhibitor). The C-EDGE coinfection and COSTAR study with this regimen have already been summarized above. Further important presentations included a pooled analysis for all GT1 patients treated in phase 2/3, a pooled analysis on all GT4 patients, as well as an integrated analysis of all patients with compensated cirrhosis with GT1, 4 or 6 infection and finally an analysis on the impact of baseline NSA resistance associated variants (RAVs) on the efficacy of Elbasvir/Grazoprevir (EBR/GZR) against GT1a infection (22-25). In phase 2-3 trials, 95% of GT1-infected patients (±cirrhosis, HIV coinfection, prior treatment, end-stage renal disease) who received grazoprevir 100 mg + elbasvir 50 mg (GZR/EBR) ± ribavirin (RBV) achieved a sustained virologic response 12 weeks after end of therapy (SVR12); 3% experienced virologic failure. This analysis assessed potential predictors of response to therapy (22). Analyses were performed on 2 pooled datasets of 1408 GT1-infected patients enrolled in phase 2/3 trials of GZR/EBR ± RBV: (1) treatment-naïve patients (TN; N=801), and (2) patients who previously failed peginterferon + RBV ± first-generation protease inhibitor (TE;N=607). Demographic factors and presence of resistance-associated variants (RAVs) that were fit (ie, >25% of the overall baseline viral load) were considered. Univariate logistic regression models were fitted one variable at a time in assessing the potential association with SVR12. Multivariable logistic regression (MVLR) models with forward selection were then applied to identify significant (ie, P < 0.1) independent predictors of SVR12. Figure 24 summarizes the main SVR12 rates by different variables for the treatment-naïve patient population.
Figure 24: SVR12 by variable in GT1a-infected patients in the treatment-naïve pooled efficacy population
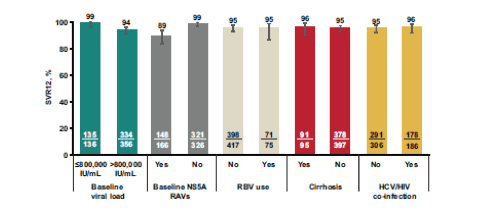
Only baseline HCV RNA >800,000 IU/mL and presence of baseline NS5A RAVs were identified as significant predictors of SVR12 in GT1a-infected TN patients. Neither of these factors predicted SVR12 in GT1b-infected TN patients. No impact was observed for RBV use or presence of HIV coinfection. The finding for the main SVR12 rates by different variables for the treatment-experienced patient population is depicted in figure 25.
Figure 25: SVR12 by variable in the treatment-experienced pooled efficacy population
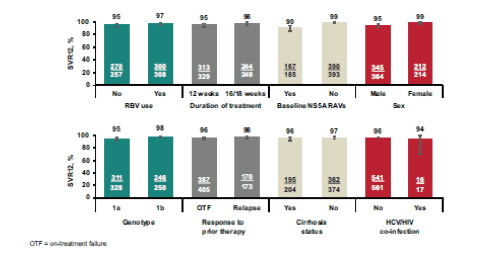
Among GT1a-infected patients, female patients and non-cirrhotics had (numerically) higher SVR12 rates. In addition use of RBV and/or longer treatment duration had a positive impact on SVR12. It is however important to highlight that in this analysis only patients with compensated cirrhosis were included so no conclusion can be made from this data for patients with more advanced cirrhosis stages. Among GT1a-infected patients, baseline NS5A RAVs had a modest negative impact on SVR12, however this impact was confined to the 12-week treatment duration, as no patient treated for 16 or 18 weeks with RBV experienced virologic failure suggesting that perhaps addition of ribavirin and extending treatment duration may be helpful in GT1a patients to increase SVR12 rates in patients with baseline NS5A RAVs and negative prediction factors.
The impact of NS5A RAVs against the combination of elbasvir/grazoprevir was further explored in a pooled analysis of all GT1a patients in a late breaker poster (25). Previous studies had demonstrated that a 12-week EBR/GZR regimen without ribavirin was highly effective in GT1a patients. Virologic failure occurred mainly among patients in whom population sequencing at baseline (BL) identified substitutions at NS5A resistance-associated positions 28, 30, 31, 58, or 93 that reduced EBR potency ≥5-fold in vitro (5XRAVs). In GT1a patients who received 16 weeks of GZR/EBR+RBV, no virological failures were observed despite the presence of 5X-RAVs. In this new study the association between presence of BL NS5A RAVs and SVR12 using a more sensitive next generation sequencing (NGS) assay on BL samples from treatment-naïve (TN) or experienced (TE) GT1a patients in Phase 3 trials was evaluated. It is important to note that with the use of deep-sequencing techniques the number of patients with baseline RAVs increases. This can have a diluting effect in the sense that the more patients are added where very low frequencies of RAVs exist, which may have no clinical impact at all, the more difficult it will become to determine whether these RAVs have an impact on SVR rates or not. In contrast RAVs picked up by population sequencing need to be present in a higher frequency in order to become detected and actually have been shown to significantly decrease probability of SVR12 after 12 weeks of therapy with elbasvir/grazoprevir without ribavirin. Therefore in clinical practice population sequencing is more likely to be the clinically relevant resistance test, which may be considered in patients with unfavorable prediction factors for achieving SVR. Figure 26 shows the distribution of NS5A RAVS for the two resistance assay techniques and also their respective impact on SVR12.
Figure 26: Efficacy of EBR/GZR in GT1a TN/prior relapsers with baseline NS5A
RAVs
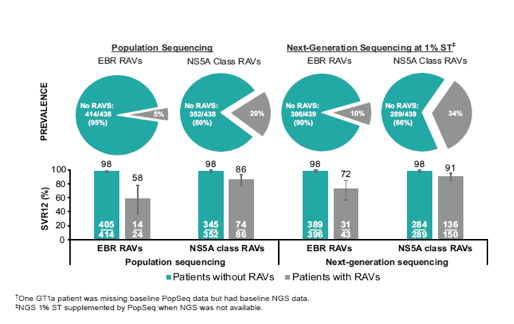
Indeed the number of elbasvir specific as well as NS5A RAVS in general increases substantially with deep sequencing. Most interestingly the detection of more RAVS is associated with less impact on SVR suggesting that indeed some of those mutations found at very low levels may be less meaningful clinically. Among GT1b TN/prior relapsers with baseline NS5A RAVs, the efficacy of EBR/GZR (12 weeks, no RBV) was high regardless of methodology once again highlighting that the role of RAVS is very different between GT1a and 1b with GT1b being not affected by baseline mutations. In a further analysis efficacy of EBR/GZR 16/18 weeks (+ RBV) was evaluated in GT1a PR non-responders with baseline NS5A RAVs which is shown in figure 27.
Figure 27: Efficacy of EBR/GZR 16/18 weeks (+ RBV) in GT1a PR non-responders with baseline NS5A RAVs.
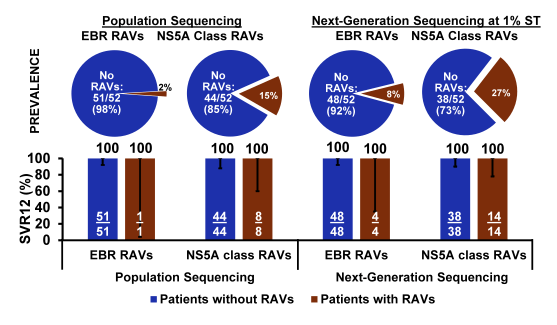
Most reassuringly, among GT1a PR (prior relapsers) non-responders with baseline NS5A RAVs, the efficacy of EBR/GZR (16/18 weeks, with RBV) was high regardless of methodology suggesting that slight treatment extension and addition of RBV may be able to overcome some of the RAVs associated impact on SVR rates.
Further interesting data on the elbasvir/grazoprevir combination derived from an analysis of the efficacy/safety of GZR/EBR ± RBV among compensated cirrhotics in the phase 2/3 GZR/EBR program. Treatment-naive (TN) and treatment-experienced (TE) pts with compensated cirrhosis received GZR/EBR ±RBV for 12 (TN) or 12-18 weeks (TE) in 6 studies (24). Overall, 402 subjects with cirrhosis were included, 65.7% were male, median age was 56y, 80.6% were white, 9.2% black, 54.5% had GT1a, 37.8 GT1b, 5.7% had GT4, HIV-coinfection 10%, 42% were treatment-naïve and 58% had prior treatment failure which was mostly PR nonresponse (37.3%). With regard to cirrhosis baseline characteristics 25% of patients had a platelet below 100.000cells/ml and 6.2% a serum albumin <3.5 g/dL. Cirrhosis diagnosis was made either on biopsy (28.6%), fibroscan (64.2%) or APRI + fibrotest (7.2%). 36% of patients had a fibroscan value above 25. Figure 28 (left figure) shows the SVR12 rates for the treatment-naïve patients receiving 12 weeks of therapy and no RBV (Full analysis set) and in the right part the SVR12 rates for the treatment experienced patients.
Figure 28: SVR12 rates for the treatment-naïve patients receiving 12 weeks of therapy and no RBV (Full analysis set) and in the right part the SVR12 rates for the treatment experienced patients.
Overall efficacy in treatment-naive patients was very high suggesting that 12 weeks are clearly enough in cirrhotic patients. As the disease characteristics are more suggestive of compensated cirrhotics the question remains unclear whether this also holds true for CHILD B and C patients. In treatment-experienced patients with GT1a infection outcome in cirrhotics can be further improved by adding ribavirin and extending to 16 or 18 weeks. GZR/EBR regimens were generally well tolerated among cirrhotic patients.
A further interesting pooled analysis from the elbasvir/grazoprevir phase 2/3 trials was presented for all genotype 4 (n=47 GT 4a; n=47 GT4d) patients (23). Overall 103 GT4 patients were analyzed, 66 patients were treatment naïve and 37 treatment experienced. The overall outcome by treatment regimen and schedule is shown below in figure 29.
Figure 29: SVR12: Full analysis set for all GT4 patients
A 12-week regimen of EBR/GZR was highly effective among 75 GT4-infected TN and prior relapse patients. Treatment outcomes did not differ according to GT4 subtype. A regimen of EBR/GZR + RBV for 16 weeks may result in improved efficacy among patients with prior treatment failure although the sample size was small.
Finally, a separate analysis evaluated the safety and tolerability of the elbasvir/grazoprevir fix dose combination (26). Aim of this study was to define the overall safety profile of EBR 50 mg/GZR 100 mg given for 8, 12, 16, or 18 weeks ± RBV in patients with HCV genotypes 1, 4, or 6 infections with and without cirrhosis. The baseline characteristics of this large dataset (n=1690) are shown in table 6 and the overall safety summary in table 7.
Table 6: Patient demographics
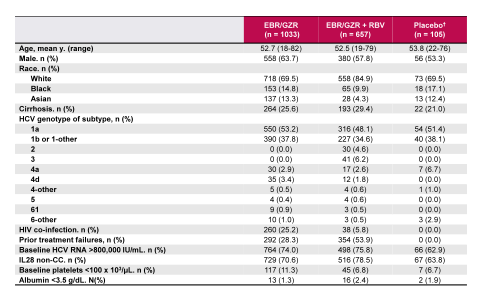
Table 7: Overall safety summary
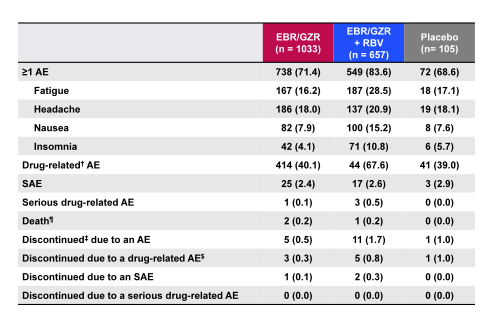
EBR/GZR ± RBV overall had a generally favorable safety profile in a large, diverse patient population, including patients with Child-Pugh A, compensated cirrhosis. Very few SAEs or discontinuations were recorded. Common adverse events such as fatigue, headache, and nausea occurred at a similar frequency on active and placebo treatments. Ribavirin containing regimens were associated with an expected increase in reported AEs and drug-related AEs. Importantly, tolerability was not affected by treatment duration or compensated cirrhosis. Elevations of ALT from normal levels to >5× ULN occurred in 0.8% of patients who received EBR/GZR and generally resolved with continued therapy or scheduled end of therapy.
AASLD: High Efficacy of Elbasvir and grazoprevir With or Without Ribavirin in 103 Treatment-Naive and Experienced Patients With HCV Genotype 4 Infection: A Pooled Analysis - (12/01/15)
AASLD: Prevalence and Impact of Baseline NS5A Resistance-Associated Variants (RAVs) on the Efficacy of Elbasvir/Grazoprevir (EBR/GZR) Against GT1a Infection - 16 Weeks vs 12 weeks - (11/23/15)
AASLD: Prevalence and Impact of Baseline NSA Resistance Associated Variants (RAVs) on the Efficacy of Elbasvir/Grazoprevir (EBR/GZR) Against GT1a Infection - (11/19/15)
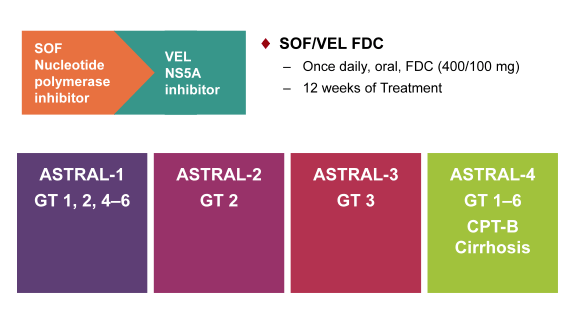
The next new DAA combination which received very much attention was the combination of sofosbuvir with velpatasvir (VEL) which is a new fix dose combination of the already well known and licensed nucleotide polymerase inhibitor sofosbuvir in combination with the novel pangenotypic NS5A inhibitor velpatasvir (GS-5816) which is considered to have a different resistance profile. Obviously, pangenotypic regimens are of great interest with regard to roll-out of DAA-based HCV treatment on a global level but also for treatment of genotype 3 as this still remains a challenging to treat GT up to now. This new DAA combination was evaluated in the ASTRAL study program in various different patient populations, which is summarized below in figure 30.
Figure 30: ASTRAL study program
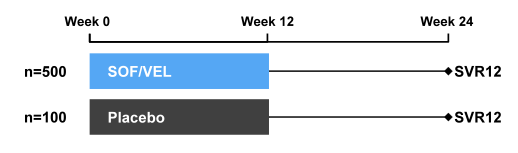
The ASTRAL-1 study was a phase 3 study which evaluated treatment with a fixed dose combination of SOF/VEL for 12 weeks in patients with genotype 1, 2, 4, 5, or 6 HCV infection (27, 28). Patients with genotype 1, 2, 4, or 6 chronic HCV infection were randomized 5:1 to received SOF/VEL (400 mg /100 mg daily) or placebo for 12 weeks. Patients with genotype 5 infection were enrolled to the SOF/VEL treatment group. Patients with genotype 3 infection were evaluated in a separate study. The study design of the ASTRAL-1 study is depicted below in figure 31. The primary efficacy analysis was an evaluation of the superiority of SVR12 for the SOF/VEL-treated patients to a pre-specified SVR12 goal of 85%. Secondary endpoints included safety/tolerability, resistance, and additional efficacy outcomes.
Figure 31: Study design of the ASTRAL 1 study
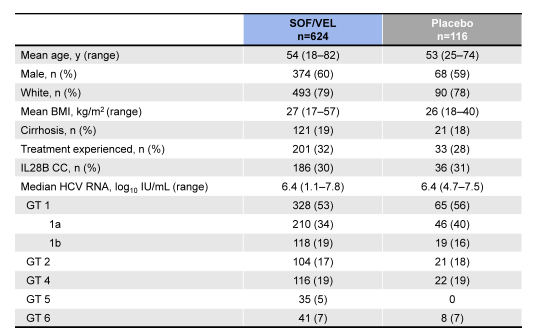
740 patients were enrolled at 81 sites in North America, Europe and Hong Kong. The baseline demographics are shown below in Table 8.
Table 8: Baseline demographics of the ASTRAL-1 study
Of note the majority of patients included had a genotype 1 infection but there was a fair amount of other genotypes included. Remember that GT3 patients were enrolled in a different ASTRAL study. 19% of patients had cirrhosis and 32% had experienced prior HCV treatment failure suggesting that there were also some more challenging to treat patients included into this trial. The main SVR outcome rates are shown below in figure 32. Overall, an impressive 99% achieved SVR12 indicating that even higher cure rates than previously observed under DAA combination therapy may be obtained with this regimen. Two of 325 patients (0.6%) with genotype 1 infection, including 1 of 73 with cirrhosis, had virologic relapse: 1 genotype 1a treatment-naïve non-cirrhotic and 1 genotype 1b treatment-experienced with cirrhosis. No patients with genotype 2, 4, 5, or 6, including 48 with cirrhosis, had virologic failure. Four patients did not achieve SVR12 for non-virologic reasons (eg. lost to follow-up).
Figure 32: ASTRAL-1: SVR12 by HCV Genotype
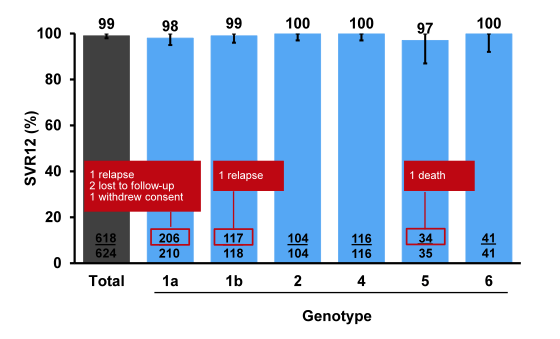
Figure 33 shows SVR rates by cirrhosis status and treatment history.
Figure 33: ASTRAL-1: SVR12 by Cirrhosis Status or Treatment History
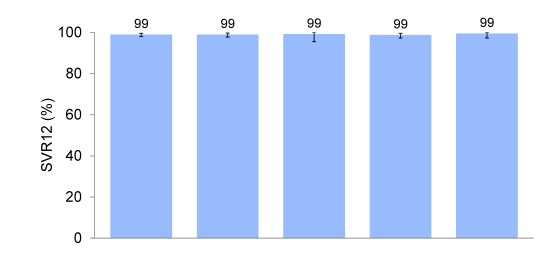
Clearly, the high response rates irrespective of disease stage and treatment history emphasize the potency of this regimen. Safety assessment in this study was possible against placebo. The main safety findings are shown in table 9.
Table 9: Safety results of the ASTRAL-1 study
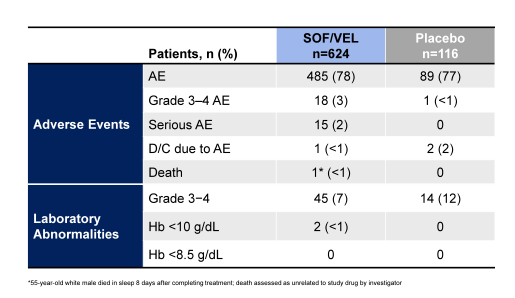
Of note the type, frequency and severity of AEs and laboratory abnormalities were similar in the SOF/VEL-treated patients compared with the 116 placebo-treated patients. Three patients discontinued treatment due to adverse events, 1 treated with SOF/VEL and 2 with placebo. One SOF/VEL-treated patient died from an unknown cause 8 days after completion of treatment. Fifteen (2.4%) SOF/VEL-treated patients and no placebo-treated patients experienced SAEs; none was assessed as related to study drug. In summary, treatment with the once daily, single tablet regimen of SOF/VEL for 12 weeks is well tolerated and results in high SVR12 rates in treatment-naïve and treatment-experienced genotype 1, 2, 4, 5, and 6 HCV-infected patients with and without cirrhosis.
AASLD: A Phase 3 Double-Blind Placebo-Controlled Evaluation of Sofosbuvir/Velpatasvir Fixed-Dose Combination for 12 Weeks in Genotype 1, 2, 4, 5, 6 HCV-Infected Patients: Results of the ASTRAL-1 Study - (11/17/15)
AASLD: Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection - (11/30/15)
The ASTRAL-2 study compared the efficacy and safety of treatment with a fixed dose combination (FDC) of SOF/GS-5816 for 12 weeks to SOF + RBV for 12 weeks in treatment-naïve and treatment-experienced genotype 2 HCV-infected patients, with and without cirrhosis (29). This study is of particular interest as real-life data at this meeting suggested that at least in treatment experienced GT2 patients with cirrhosis SVR rates were only slightly above 80% (see also above) so indicating that there is room for improvement. Also treatment alternatives are needed for patients who are intolerant to ribavirin. Enrolled patients were randomized 1:1 to receive either SOF/VEL (400 mg /100 mg daily) FDC for 12 weeks or SOF
(400mg daily) with RBV (1000-1200mg daily) for 12 weeks. Randomization was stratified by prior treatment and cirrhosis status. The study design is depicted below in figure 35.
Figure 35: Study design of the ASTRAL-2 study
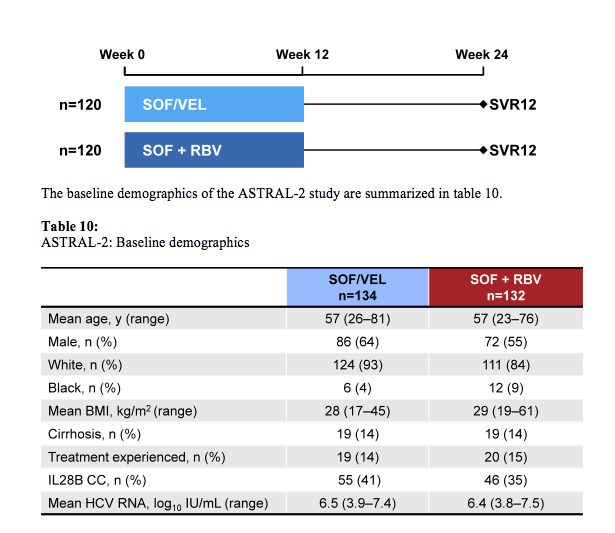
Of note only 14% of patients had cirrhosis at baseline and only 14-15% were treatment experienced limiting the conclusions from this particular patient group. Overall SVR12 rates are shown below in figure 36.
Figure 36: ASTRAL-2: SVR12 by Treatment
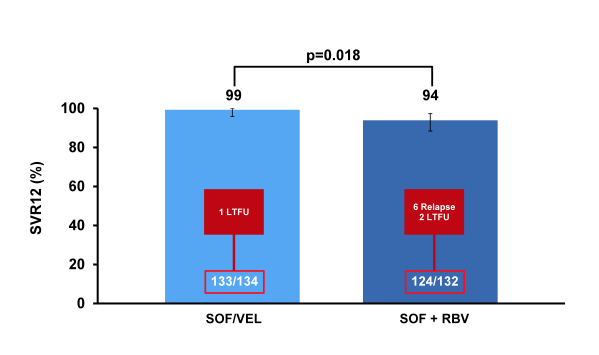
Overall, response rates were high for both treatment groups reflecting the relatively favorable treatment response predictive factors. Most impressively, however no relapse was noted in the SOF/VEL treated group but 6 relapses in the SOF/RBV group. Overall safety was good with fatigue and insomnia being more common in patients treated with SOF + RBV. In conclusion, treatment with SOF/VEL FDC for 12 weeks resulted in high SVR12 rates and was well tolerated in treatment-naïve and treatment-experienced genotype 2 HCV-infected patients with and without cirrhosis (but caveat only few cirrhotics included. Few (<1%) patients discontinued treatment and among adverse events fatigue and insomnia were more common in patients treated with SOF + RBV. Clearly, this new DAA combination has potential to become a preferred regimen for treatment of GT2 patients. More treatment outcome data in more advanced fibrosis stages are needed.
AASLD: A Randomized Controlled Trial of Sofosbuvir/Velpatasvir Fixed-Dose Combination for 12 Weeks Compared to Sofosbuvir with Ribavirin for 12 Weeks in Genotype 2 HCV-Infected Patients: The Phase 3 ASTRAL-2 Study - (11/30/15)
AASLD: Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection - (11/30/15)
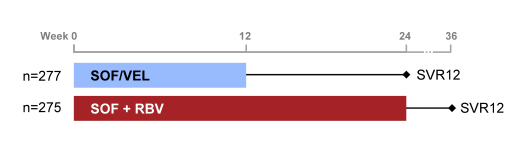
The ASTRAL-3 study was a phase 3 study comparing treatment with a fixed dose combination (FDC) of SOF/VEL for 12 weeks to standard of care, SOF+RBV for 24 weeks, in patients with genotype 3 HCV (30). As GT 3 treatment remains the biggest challenge in DAA HCV therapy this new regimen with the inclusion of a proposed pangenotypic NS5A inhibitor was awaited with great expectation. Patients at 75 sites in North America, Europe, Australia and New Zealand were randomized 1:1 to receive SOF/VEL (400 mg /100 mg daily) FDC for 12 weeks or SOF (400mg daily) with RBV (1000-1200mg daily) for 24 weeks. The study design is shown below in figure 37.
Figure 37: ASTRAL-3 study design
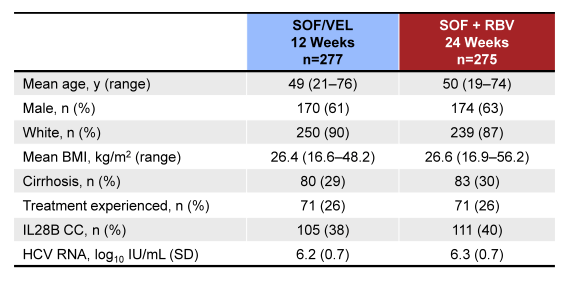
Of note this was a large study with 552 GT3 subjects included. The baseline demographics are shown below in Table 11.
Table 11: ASTRAL-3: Baseline demographics
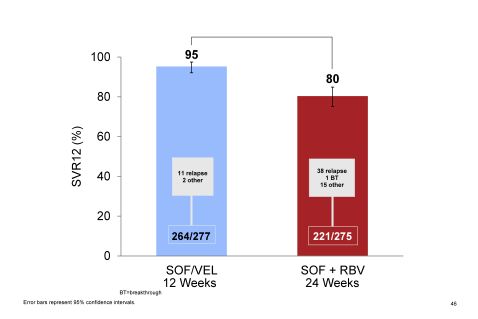
It is important to highlight that this study did include a substantial proportion of cirrhotics and prior treatment failures enabling to assess efficacy of this new regimen in a more difficult to treat patient population. The overall SVR 12 rates for the two study groups are shown in figure 38.
Figure 38: ASTRAL-3 study: SVR12 rates by treatment group
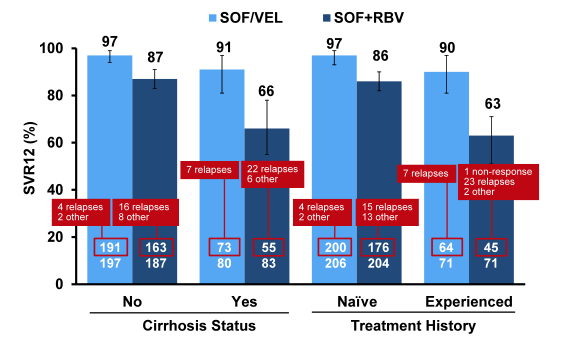
Clearly, more relapses were observed in the standard of care sofosbuvir + ribavirin group. SVR12 rates with respect to baseline cirrhosis or treatment failure are shown in figure 39.
Figure 39: ASTRAL-3: SOF/VEL FDC Daily for 12 Weeks versus SOF/RBV for 24 Weeks in Patients with HCV Genotype 3 Infection: Impact of baseline cirrhosis or prior treatment failure.
Notably, SVR12 rates were higher in all groups for the SOF/VEL treatment arm. As in other clinical trials SVR12 rates dropped quite substantially in the sofosbuvir + ribavirin for 24 weeks group again underlining the need for better treatment options in this group. Although the 12 week arm of SOF/VEL clearly outperformed the control arm there were more relapses than noted in other ASTRAL trials for different genotypes reminding us that GT3 was and is a difficult to treat genotype. Clearly the question arises which role potentially addition of ribavirin or longer treatment durations would have in cirrhotic and treatment experienced gT3 patients. With regard to safety analysis six patients in the SOF/VEL treatment group and 15 patients in the SOF+RBV treatment group experienced SAEs. One SAE, acute generalized exanthematous pustulosis, in a SOF+RBV treated patient was assessed as related to study drugs by the investigator. Hemoglobin decline and total bilirubin increases were more commonly observed in the group treated with SOF +RBV consistent with RBV-induced hemolysis. No other significant lab abnormalities were observed. In summary, the combination of SOF/VEL led to higher SVR12 rates than 24 weeks of SOF/RBV and was very well tolerated.
AASLD: Sofosbuvir/Velpatasvir Fixed-Dose Combination for 12 Weeks Compared to Sofosbuvir with Ribavirin for 24 Weeks in Genotype 3 HCV-Infected Patients: The Randomized Controlled Phase 3 ASTRAL-3 Study - (11/30/15)
AASLD: Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection - (11/30/15)
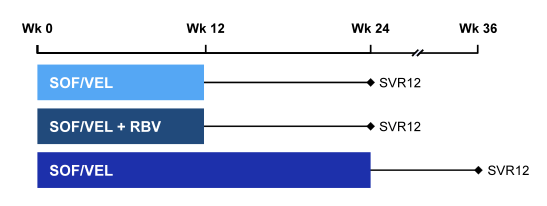
The ASTRAL-4 study was a phase 3 study which evaluated the safety and efficacy of the fixed dose combination (FDC) of SOF/VEL in HCV infected patients with decompensated liver disease (31). Obviously, this is among the most challenging to treat patient population and therefore this study can be regarded as a stress test for this new DAA combination. Genotype (GT) 1, 2, 3, 4 or 6 HCV infected patients with CPT-B cirrhosis were randomized 1:1:1 to receive SOF/VEL (400 mg /100 mg) daily for 12 weeks, SOF/VEL + weight based RBV for 12 weeks, or SOF/VEL for 24 weeks. Patients with prior liver transplant or hepatocellular carcinoma were excluded. By adding ribavirin into one arm also the additional role of ribavirin was going to be assessed within this study. Figure 40 shows the study design.
Figure 40: ASTRAL-4 study design

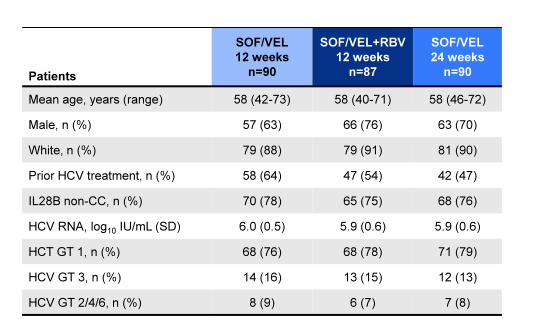
The baseline demographics of the ASTRAL-4 study are shown below in Table 12. More than half of the patients were treatment experienced and more than three quarters were GT1 infected.
Table 12: ASTRAL-4 baseline demographics
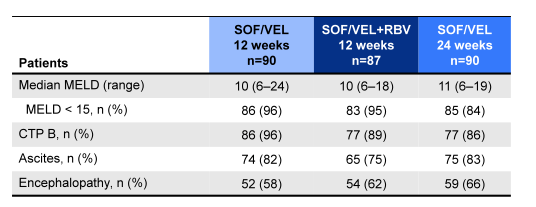
Liver disease characteristics are summarized in table 13. The high rate of ascites and hepatic encephalopathy underscores the very advanced liver disease stage of the patients included into the trial.
Table 13: Liver disease characteristics
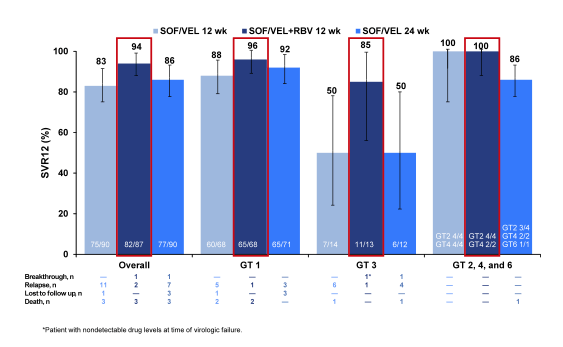
The SVR12 rates for the different treatment arms are shown in figure 41. SOF/VEL+RBV for 12 weeks resulted in the highest SVR rates with relapse occurring in only 1 (1%) GT1 and 1 (8%) GT3 subjects respectively. A second GT3 patient in the SOF/VEL+RBV 12 Week group had an on-treatment breakthrough with pharmacokinetic data consistent with nonadherence. Obviously, the addition of ribavirin was the best additive intervention to increase SVR rates rather than just extending treatment duration to 24 weeks. There were no genotype 2, 4 and 6 virologic failures across all treatment arms. Among patients who achieved SVR, 47% and 56% had improvements in CPT and MELD scores by week 12 post EOT largely driven by increases in albumin and decreases in bilirubin.
Figure 41: ASTRAL-4: SOF/VEL ± RBV in HCV patients with decompensated liver disease: SVR12 rates overall and by genotype.
The most common adverse events were fatigue, headache, nausea and (anemia in the RBV containing arm with a median Hgb decrease of 1.4 g/dL). Overall 9 patients discontinued SOF/VEL due to adverse events. A total of 47 (18%) patients experienced serious adverse events (SAEs) with the most common being hepatic encephalopathy and sepsis; only 1 patient had SAEs assessed as related to SOF/VEL There were 9 deaths: sepsis (3); liver failure (2); cardiopulmonary arrest (1); myocardial infarction (1) and respiratory failure (1); none were assessed as related to study drug. It is important to remember that these patients with predominantly CHILD B cirrhosis are already very sick and risk of complications from the underlying liver disease is high. In summary, in HCV infected patients with decompensated liver disease, SOF/VEL+RBV for 12 weeks resulted in an overall SVR rate of 94.3% with high individual SVR rates across all HCV genotypes and resulted in early improvements in liver function in more than 50% of patients. This regimen was well tolerated with AEs consistent with clinical sequelae of decompensated liver disease and RBV. In this particularly challenging patient group there appears to be a beneficial role for the addition of ribavirin to the 12 weeks of SOF/VEL.
AASLD: Sofosbuvir/Velpatasvir Fixed-Dose Combination for the Treatment of HCV in Patients With Decompensated Liver Disease: the Phase 3 ASTRAL-4 Study / ASTRAL 1, 2 and 3 - (11/30/15)
AASLD: Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis (ASTRAL-4) - (11/30/15)
Another new DAA combination which is a little bit further behind in development than the two previously described new DAA is the combination of the next-generation HCV DAA ABT-493, an NS3/4A protease inhibitor identified by AbbVie and Enanta, and ABT-530, an NS5A inhibitor. Both compounds are characterized by potent pangenotypic in vitro antiviral activity against all major HCV genotypes (GTs), including activity against key known resistance-associated variants and a high barrier to resistance selection. Indeed monotherapy with ABT-493 or ABT-530 resulted in a mean 4 log10 IU/mL decline from baseline in HCV plasma viral load in GT1-infected subjects with and without compensated cirrhosis. At AASLD the results of the phase 2 study (SURVEYOR) was presented which evaluated treatment with ABT-493 and ABT-530 for 12 weeks in HCV GT1-infected subjects without cirrhosis (32). The study design is shown below in figure 42.
Figure 42: Study design of the SURVEYOR-1 study
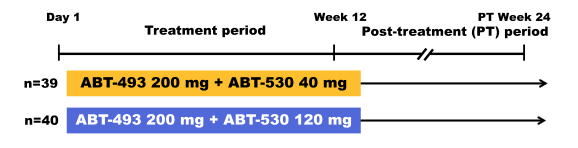
Non-cirrhotic GT1-infected treatment-naïve (TN) or pegylated interferon/ribavirin (pegIFN/RBV) null responder subjects received once-daily ABT-493 200 mg + ABT-530 120 or 40 mg for 12 weeks, and subsequently were followed for 24 weeks. 79 subjects (male, 52%; median [range] age, 54.0 [26.0-70.0] years; GT1a, 81%; GT1b, 19%; TN, 63%; pegIFN/RBV null responders, 37%; fibrosis >F2, 25%; median [range] HCV RNA log10 IU/
mL, 6.8 [4.4-7.5]) were enrolled, 40 received ABT-493 200 mg+ABT-530 120 mg and 39 received ABT-493 200 mg and ABT-530 40 mg. The SVR12 outcome is shown in figure 43.
Figure 43: SVR12 results for the SURVEYOR-1 study
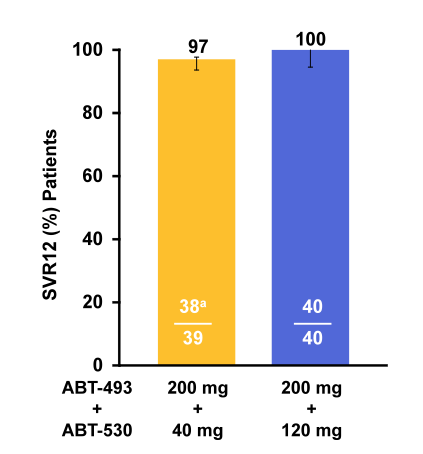
Once-daily 12-week treatment with the next-generation HCV DAAs ABT-493 and ABT-530 of GT1 infection in non-cirrhotic TN and pegIFN/RBV null responders resulted in high SVR12 (97%-100%) rates. Overall, only one treatment relapse was observed in the low dose treatment arm. That individual had F0-F1 fibrosis, was treatment naïve and had no baseline NS5A mutations. Upon relapse the following NS5A mutations became detectable: Q30K + H58D. Based on these encouraging results, the SURVEYOR-1 study with once-daily ABT-493 and ABT-530 has been expanded to include GT1 subjects with compensated cirrhosis and evaluate a shorter, 8 week treatment duration in non-cirrhotic subjects. Clearly, more data is needed to better judge on the efficacy of this regimen but clearly these first results are extremely encouraging.
AASLD: SURVEYOR-I: 98% - 100% SVR4 in HCV Genotype 1 Non-Cirrhotic Treatment-Naïve or Pegylated Interferon/Ribavirin Null-Responders with the Combination of the Next Generation NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 - (11/16/15)
AASLD: 100% SVR4 in HCV Genotype 1 Non-Cirrhotic Treatment-Naïve or -Experienced Patients With the Combination of ABT-493 and ABT-530 for 8 Weeks (SURVEYOR-I)....[8 weeks] - (11/17/15)
AASLD: Analysis of HCV Genotype 1 Variants Detected During Monotherapy and Combination Therapy With Next Generation HCV Direct-Acting Anti viral Agents ABT-493 and ABT-530 "........COMBINATION THERAPY (SURVEYOR-I PART 1, PHASE 2 DOSE-RANGING STUDY) .....All patients with baseline NS3 or NS5A variants achieved SVR12 (Study SVR12: 99%)" - (11/18/15)
EASL: Potent Antiviral Activity of ABT-493 and ABT-530 With 3-Day Monotherapy in Patients With and Without Compensated Cirrhosis With Hepatitis C Virus (HCV) Genotype 1 Infection - (05/04/15)
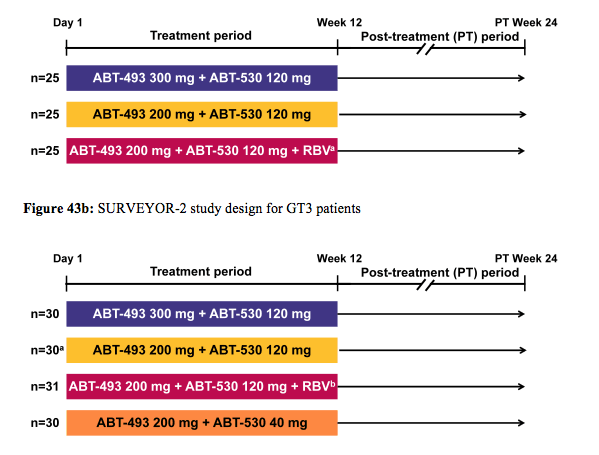
The SURVEYOR-2 study evaluated the efficacy and safety of different doses of ABT-493 and ABT-530 with or without ribavirin (RBV) in non-cirrhotic GT2 or GT3-infected treatment-naïve (TN) and pegylated interferon/RBV (pegIFN/RBV) treatment experienced (TE) subjects (33,34). The study design for the GT2 patients is shown in figure 43a and the study design for the GT3 patients in figure 43b.
Figure 43a: SURVEYOR-2 study design for GT2 patients
In the GT2 study subjects received 12 weeks of ABT-493 300 mg+ABT-530 120 mg (Arm A), ABT-493 200 mg+ABT-530 120 mg (Arm B), or ABT-493 200 mg+ABT-530 120 mg+RBV (Arm C). DAAs were dosed once daily; weight-based RBV (1000 or 1200 mg) was dosed twice daily. Subjects were then followed for 24 weeks. 75 subjects were treated in Arms A-C (n=25 each); 74 had GT2, and 1 subject initially randomized to Arm B was determined to have GT3a infection. Subjects were male, 63%; median (range) age, 57.0 (20.0-69.0) years; GT2b, 81%; TN, 88%; TE, 12%; F0-F2, 87%; F3, 13%; median (range) baseline HCV RNA log10 IU/mL, 7.1 (4.7-7.8). No subjects have experienced virologic failure. One subject in Arm A prematurely discontinued study drugs and is lost to follow-up. The SVR 12 rates for this sub-study are shown on figure 44a.
Figure 44a: SURVEYOR-II Part 1 (GT2): ITT SVR12 Rates by Treatment
Clearly, the response rates in this GT2 patient population are impressive and again could be important for patients who cannot tolerate ribavirin and are waiting for HCV treatment options beyond sofosbuvir and ribavirin.
AASLD: SURVEYOR-II: High SVR4 Rates Achieved With the Next Generation NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 in Non-Cirrhotic Treatment-Naïve and Treatment-Experienced Patients With HCV Genotype 2 Infection - (12/01/15)
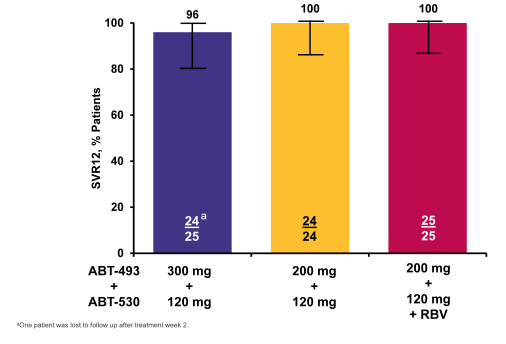
In the GT3 part of the SURVEYOR2 study subjects received 12 weeks of ABT-493 300 mg+ABT-530 120 mg (Arm D), ABT-493 200 mg+ABT-530 120 mg (Arm E), ABT-493 200 mg+ABT-530 120 mg+RBV (Arm F), or ABT-493 200mg+ABT-530 40 mg (Arm G) (34). DAAs were dosed once daily; weight-based RBV (1000 or 1200 mg) was dosed twice daily. The primary efficacy endpoint was sustained virologic response 12 weeks after the last dose of study drug (SVR12). 120 GT3-infected subjects were treated in Arms D (n=30), E (n=29), F (n=31), or G (n=30). Subjects were male, 56%; median age, 52.0 years; GT3a, 98%; TN, 92%;TE, 8%; fibrosis >F2, 15%; median baseline HCV RNA log10 IU/mL, 6.7. The SVR12 rates are shown below in figure 44b. There has been only 1 virologic failure in the group of patients receiving the highest dose combination of ABT-493 300mg + ABT-530 120mg and also only 1 virological failure in the arm with additional ribavirin. 2 and 3 virological failures were seen in the other two arms with the lower dose of either ABT-493 or ABT-530, respectively. The patient failing in the high dose arm had no NS3 mutations and one NS5A (A30K) at baseline and at relapse a double NS3 and NS5A variant was found. One subject in Arm G was lost to follow-up at the week 2 visit. AEs were m mostly mild, with most common DAA-related AEs being fatigue, nausea, and headache. There were no serious DAA-related AEs; 1 subject discontinued due to DAA- and RBV-related AEs of abdominal pain and heat sensation. Typical reductions in hemoglobin were observed in the RBV containing arm.
Figure 44b: SURVEYOR-II Part 1 (GT3): ITT SVR12 Rates by Treatment
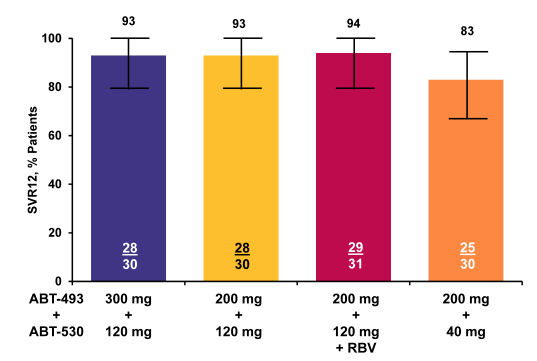
Overall, efficacy was very good with SVR rates between 83 and 94%. Lowest response rate was in the lowest dose arm of ABT-530. Addition of ribavirin did not seem to increase SVR rate indicating that this regimen may not require ribavirin. More data is however needed to answer this question.
AASLD: SURVEYOR-II: High SVR4 Rates Achieved With the Next Generation NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 in Non-Cirrhotic Treatment-Naïve and Treatment-Experienced Patients With HCV Genotype 3 Infection - (12/01/15)
Finally, at AASLD a whole range of 3 DAA combinations were studied. 3 DAA combinations are of great interest as they may allow shorter treatment durations and also protect better against resistance development. The first combination studied to report back on was well-known sofosbuvir (SOF) (nucleotide polymerase inhibitor), velpatasvir (former GS-5816; NS5A-inhibitor), and GS-9857 (NS3-inhibitor) (35). The combination of these three pangenotypic agents has the potential to improve efficacy and may reduce treatment duration across different patient populations. At this year AASLD results were presented from a study evaluating the safety and efficacy of this regimen administered for 6 or 8 weeks in more difficult-to-treat populations, including treatment experienced (TE) GT 1 patients with cirrhosis who had failed PEG/RBV; TE GT 1 patients who had failed protease inhibitor (PI) + PEG/RBV; TN GT 3 patients with cirrhosis; and TE GT 3 patients with cirrhosis who had failed PEG/RBV (35). All patients received once daily SOF/VEL 400mg/100mg in a fixed dose combination, plus GS-9857 100mg. Treatment duration of each group differed according to the patient's baseline characteristics: 8 weeks for TE GT 1 patients who had failed prior PEG/RBV, 8 weeks for TE GT 1 patients who had failed PI + PEG/RBV, 6 weeks for TN GT 3 patients and 8 weeks for TE GT 3 patients. The corresponding SVR12 rates for the different patient populations and treatment durations are shown in figure 45. Impressively, almost all 8 week treated patients (GT 1 or 3 TE with cirrhosis) achieved SVR12 whereas few relapses were noted in the arm with the shorter treatment duration as well as in the arm with 8 weeks therapy in GT1 cirrhotics with previous treatment failure including a PI based HCV regime.
Figure 45: SOF/VEL + GS-9857: SVR12 Rates by GT and treatment duration.
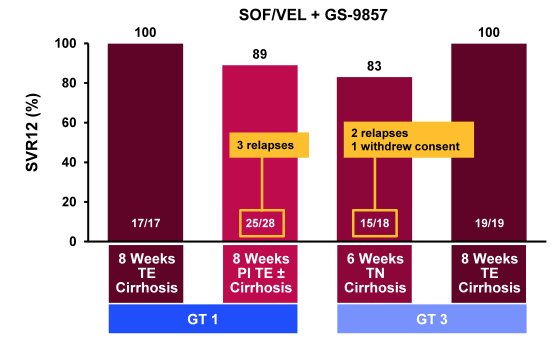
Under consideration that these were extremely difficult to treat patients (cirrhosis and previous treatment failure) these results are very impressive. Indeed this is one of the first DAA studies to report 100% SVR12 in GT3 patients with cirrhosis and prior treatment failure. Further subanalysis suggested that baseline NS5A and NS3 RAVs had an impact on SVR12 rates (90% vs 95% for baseline NS5A RAVS present vs not present). With regard to safety no patient discontinued study treatment. Adverse events were generally mild, and Grade 3/4 laboratory abnormalities were infrequent. The most frequent adverse events were headache (22%), nausea (21%), and fatigue (17%).
AASLD: Sofosbuvir/Velpatasvir + GS-9857 for 6 or 8 Weeks in Genotype 1 or 3 HCV-Infected Patients - (11/16/15)
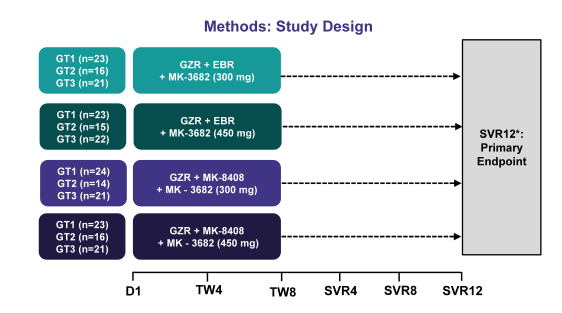
The other interesting 3 DAA combination which was presented was the combination of grazoprevir, an NS3/4A protease inhibitor; MK-3682, an NS5B polymerase inhibitor, and either elbasvir or MK-8408, which are NS5A inhibitors. In this first trial efficacy and safety of this new 3 DAA combination was evaluated (36). In Part A of 2 ongoing randomized, dose-ranging, parallel-group, multicenter, open-label Phase 2 trials, 93 GT1 (46 GT1a, 47 GT1b), 61 GT2, and 86 GT3-infected treatment-naïve, non-cirrhotic patients with chronic HCV infection were dosed once-daily for 8 weeks duration with one of 4 regimens including grazoprevir (100 mg), MK-3682 (300 mg or 450 mg), and either elbasvir (50 mg) or MK-8408 (60 mg). The study design for this study is shown in figure 46.
Figure 46: Study design
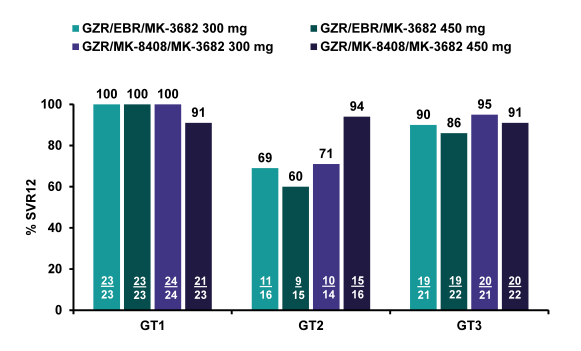
Overall, 240 HCV-infected treatment-naïve, non-cirrhotic individuals were included. Across treatment arms, 45/46 (98%) GT1a and 46/47 (98%) GT1b patients achieved sustained virologic response 12 weeks after end of study therapy (SVR12) (see figure 47). In the 2 relapsers, population sequencing did not detect NS3, NS5B, or NS5A resistance associated variants (RAVs) conferring ≥5-fold potency shifts at baseline or following relapse. The grazoprevir/MK-3682 (450 mg)/MK-8408 regimen was also highly effective in GT2 patients, with SVR12 among 15/16 (94%) of GT2-infected patients, but regimens containing the 300 mg dose of MK-3682 and/or elbasvir resulted in lower efficacy (SVR12 in 29/45 (64%) GT2 patients; 60-71% across the 3 arms). Relapses were more common among patients who harbored an L31M/I NS5A variant at baseline. No treatment-emergent RAVs were observed. In the GT3 patients high SVR rates were achieved with 78/86 (91%) of GT3-infected patients reaching SVR12; response was comparable across arms (86-95%). Eight GT3 patients relapsed which was higher than the relapse rate observed for GT1 suggesting that GT3 overall, may still be somewhat more challenging to treat. SVR12 was lower among GT3-infected patients who harbored an NS5A A30K, L31M, or Y93H RAV at baseline compared
with patients without these RAVs at baseline (5/11 [45%] vs 72/74 [97%], respectively). Two of 8 GT3 relapsers acquired NS5A Y93H. With regard to safety and tolerability it is important to note that all regimens were generally well tolerated, and no cardiac or renal safety signals were identified. The most frequent study drug-related AEs in >5% of all patients were headache, fatigue, nausea, diarrhea, flatulence and insomnia. There were no drug-related serious adverse events and no patients discontinued due to adverse events. In, conclusion this 3 DAA combination also achieved high SVR rates in the majority of patients with an eight week treatment regimen. Baseline NS5A mutations have impact on SVR rates with this 3-drug regimen in GT-3 patients.
Figure 47: Comparison of 2 Doses of Nuc (MK-3682) and 2 Different NS5A Inhibitors (Elbasvir vs. MK-8408): SVR12 rates by regimen
AASLD: Phase 2, Randomized, Open-Label Clinical Trials of the Efficacy and Safety of Grazoprevir and MK-3682 (NS5B Polymerase Inhibitor) with Either Elbasvir or MK-8408 (NS5A Inhibitor) in Patients with Chronic HCV GT1, 2 or 3 1nfection (Part A of C-CREST-1 & 2) - (11/23/15)
In summary, the 3 DAA combinations presented at AASLD increase the hope of pangenotypic regimens, which also have clear activity in GT3 patients. Also shorter treatment durations of 8 weeks appear feasible which holds the promise of easy regimens for patients with adherence challenges. Clearly the future of HCV drug regimens still has room for optimization and we will continue to see efforts in further optimizing HCV therapy beyond what is already available today.
Summary
· Treatment of acute hepatitis C with shorter durations of sofosbuvir + ribavirin is associated with low HCV cure rates; DAA combination studies promise better response rates after short treatment cycles but need to be further evaluated.
· Sofosbuvir plus ledipasvir for 8 weeks in mostly GT1 patients achieved excellent response rates in real-life settings. HIV coinfected patients responded as well as HCV monoinfected patients. High SVR rates were even seen in patients treated "outside recommendation" (HVL, F4, prior treatment, GT4). Overall, therapy with ledipasvir plus sofosbuvir was well tolerated. Shorter treatment durations of 8 week should be further explored particularly when more patients will become treated without significant fibrosis in the near future.
· HCV cure rates in clinical practice following all oral DAA combination therapy (2 DAAs +/- RBV) remain high and suggest that even in real-life patients the high HCV SVR rates (around 90% or higher) are reproducible. Patients with cirrhosis, low platelets, male gender and treatment outside the FDA guidelines have a higher risk for virological failure.
· Sofosbuvir and ribavirin lead to lower cure rates in real-life cohorts such as TARGET compared to clinical trials, which may be attributable to a higher proportion of treatment experienced patients with cirrhosis. The low response rates in GT3 patients underline the need for optimized treatment strategies in this patient group.
· Adding ribavirin to DCV + SOF for 12 or 16 weeks helps to significantly improve SVR12 rate in GT3 infected patients with advanced fibrosis or compensated cirrhosis. Whether extending treatment duration to 24 weeks further improves SVR rate cannot be answered at this moment.
· In the European compassionate use program DCV in combination with SOF±RBV for mostly 24 weeks led to high SVR12 rates in HCV GT3-infected patients, regardless of cirrhosis status, and was well tolerated. The use of RBV did not affect efficacy outcomes. However, the role of ribavirin in this patient segment can only be answered by a randomized clinical trial.
· In ALLY-2 8 weeks of therapy with daclatasvir and sofosbuvir was associated with a comparatively low SVR rate of 76%. Dosing of DCV with 30 mg in HIV-HCV coinfected patients receiving DRV/r may have contributed to cases of virologic failure, however the data suggest that low DCV systemic exposure was not the primary cause of relapse in ALLY-2 patients receiving DCV 30 mg with DRV/r. Relapse among patients receiving DRV/r with 30 mg DCV in ALLY-2 may be associated primarily with shorter (8-week) treatment duration and high baseline HCV RNA.
· High rates of SVR were achieved in patients with HCV GT1, 4 and 6 and HIV coinfection receiving the all-oral, fixed-dose combination of EBR/GZR. The HCV cure rate was comparable to the response rates to other EBR/GZR studies in HCV mono-infected patients.
· The results of the C-EDGE COSTAR study data indicate that GZR/EBR is safe and highly effective in patients with chronic HCV GT1, 4, or 6 receiving OAT (Opioid Agonist Therapy), and supports enhanced efforts to address barriers to HCV treatment access for PWID.
· The combination of ombitasvir, paritaprevir boosted with ritonavir, and dasabuvir in CHILD A patients with an HCV GT1b infection was associated with no safety signals and SVR12 was 100% emphasizing that this regimen requires no additional ribavirin in CHILD A cirrhotic patients with GT1b infection.
· Among GT1a-infected patients treated with elbasvir/grazoprevir baseline NS5A RAVs had a modest negative impact on SVR12, however this impact was confined to the 12-week treatment duration, as no patient treated for 16 or 18 weeks with RBV experienced virologic failure suggesting that perhaps addition of ribavirin and extending treatment duration may be helpful in GT1a patients to increase SVR12 rates in patients with baseline NS5A RAVs and negative prediction factors.
· The number of elbasvir specific as well as NS5A RAVS in general increases substantially with deep sequencing (versus population sequencing). Most interestingly the detection of more RAVS is associated with less impact on SVR suggesting that indeed some of those mutations found at very low levels may be less meaningful clinically. Therefore, in clinical practice population sequencing may be more meaningful in predicting potential non-response.
· In phase 2/3 studies, GZR/EBR for 12 weeks resulted in high efficacy among cirrhotic TN and GT1b-infected TE pts. For GT1a/4/6 TE pts, a 16/18-week regimen ± RBV, was highly effective. GZR/EBR regimens were generally well tolerated among cirrhotic patients.
· Treatment with the once daily, all oral, single tablet regimen of SOF/VEL (GS-5816) for 12 weeks is well tolerated and results in high SVR12 rates in treatment-naïve and treatment-experienced genotype 1, 2, 4, 5, and 6 HCV-infected patients with and without cirrhosis (ASTRAL-1 study).
· Treatment with SOF/VEL FDC for 12 weeks resulted in high SVR12 rates and was well tolerated in treatment-naïve and treatment-experienced genotype 2 HCV-infected patients with and without cirrhosis (but caveat only few cirrhotics included). Few (<1%) patients discontinued treatment and among adverse events fatigue and insomnia were more common in patients treated with SOF + RBV. Clearly, this new DAA combination has potential to become a preferred regimen for treatment of GT2 patients. More treatment outcome data in more advanced fibrosis stages are needed.
· Treatment with SOF/VEL for 12 weeks resulted in higher SVR12 rates than 24 weeks of SOF/RBV (GT3). The DAA combination was well tolerated.
· In HCV infected patients with decompensated liver disease, SOF/VEL+RBV for 12 weeks resulted in an overall SVR rate of 94.3% with high individual SVR rates across all HCV genotypes and resulted in early improvements in liver function in more than 50% of patients. This regimen was well tolerated with AEs consistent with clinical sequelae of decompensated liver disease and RBV. In this particularly challenging patient group there appears to be a beneficial role for the addition of ribavirin to the 12 weeks of SOF/VEL.
· Once-daily 12-week treatment with the next-generation HCV DAAs ABT-493 and ABT-530 of GT1 infection in non-cirrhotic TN and pegIFN/RBV null responders resulted in high SVR12 (97%-100%) rates.
· Once-daily 12-week treatment with the next-generation HCV DAAs ABT-493 and ABT-530 of GT2 or 3 infections in non-cirrhotic TN and pegIFN/RBV null responders resulted in high SVR12 rates. Whereas no relapses occurred in the GT2 sub-study few relapses were noted in the GT3 patient population.
· Once daily SOF/VEL 400mg/100mg in a fixed dose combination, plus GS-9857 100mg for 8 weeks was highly efficacious for GT1 and 3 patients with cirrhosis and prior treatment failure. The regimen was safe and well tolerated.
· An 8-week regimen of grazoprevir/MK-3682 (450 mg)/MK-8408 was highly effective and well-tolerated in GT1, 2, and 3-infected treatment-naïve, non-cirrhotic patients. Baseline NS5A mutations appear to impact SVR rates of GT3 patients.
66th Annual Meeting of the American Association for the Study of Liver Diseases
Boston, MA Nov 13-17 2015
HEPDART 2015: Frontiers in Drug Development for Viral Hepatitis
December 6-10 2015
Wailea, HI
References
1. Naggie S et al.: Sofosbuvir plus ribavirin without interferon for treatment of acute hepatitis C virus infection in HIV-1.infected Individuals (SWIFT-C). 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 1094
2. Martinello M et al. Sofosbuvir and ribavirin for six weeks is not effective among people with acute and recently acquired HCV infection: The DARE-C II Study. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 7-11, 2015, San Francisco, USA; abstract 1083
3. Fierer D et al. Sofosbuvir in the treatment of acute HCV infection in HIV-infected men. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 1090
4. Basu P et al.: Sofosbuvir and ledipasvir versus sofosbuvir and simeprevir combination therapy in the management of acute hepatitis C: A randomized open label prospective clinical pilot study. SLAM C study. Interim data. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 1074
5. Curry MP et al.: Effectiveness of 8 or 12 week LDV/SOF in treatment-naïve patients with non-cirrhotic, genotype 1 Hepatitis C: Real-world experience from the TRIO Network. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 1046
6. Christiansen S et al.: Sofosbuvir and ledipasvir for 8 weeks (SL8) in patients with hepatitis C virus (HCV) mono-infection and human immunodeficiency virus (HIV)-HCV co-infection with genotype (GT) 1 and 4 in clinical practice - results from the German hepatitis C cohort (GECCO). 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 1081
7. Terrault N et al.: Treatment outcomes with 8, 12 and 24 week regimens of Ledipasvir/Sofosbuvir for the treatment of hepatitis C Infection: Analysis of a multicenter prospective, observational Study. 66th AASLD; San Francisco, CA; November 13-17, 2015; Abstract 94.
8. Afdhal NH et al.: Failure with all-oral DAA regimens: Real-world experience from the TRIO Network. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract LB-17
9. Welzel T et al.: Safety and efficacy of sofosbuvir and ribavirin for the treatment of HCV genotype 2 and 3: Results of the HCV-TARGET Study. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 1057
10. Nelson DR et al.: ALLY-3 Study Team. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015 Apr;61(4):1127-35.
11. Leroy V et al.: All-oral treatment with daclatasvir plus sofosbuvir plus ribavirin for 12 or 16 Weeks in
HCV genotype 3-infected patients with advanced fibrosis or cirrhosis: The ALLY-3+ Phase 3 Study. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract LB-3
12. Welzel T et al.: Safety and efficacy of daclatasvir plus sofosbuvir with or without ribavirin for the treatment of chronic HCV genotype 3 infection: Interim results of a multicentre European compassionate use program. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 37
13. Wyles DL, et al.: ALLY-2 Investigators. Daclatasvir plus Sofosbuvir for HCV in Patients Coinfected with HIV-1. N Engl J Med. 2015 Aug 20;373(8):714-25.
14. Gandhi Y, et al. 16th International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy;May 26-28, 2015; Washington DC, USA. Poster 80.
15. Garimella T, et al.: Daclatasvir exposure alone does not explain HCV relapse in HIV-HCV coinfected patients receiving daclatasvir plus sofosbuvir with ritonavir-boosted darunavir in the ALLY-2 Study. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 728
16. Rockstroh JK, et al.: High efficacy of Grazoprevir/Elbasvir (GZR/EBR) in HCV genotype 1, 4, and 6-infected patients with HIV coinfection: SVR24 data from the Phase 3 C-EDGE Coinfection Study. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 210
17. Rockstroh JK ,et al.: Efficacy and safety of grazoprevir (MK-5172) and elbasvir (MK-8742) in patients with hepatitis C virus and HIV co-infection (C-EDGE CO-INFECTION): a non-randomised, open-label trial. Lancet HIV. 2015 Aug;2(8):e319-27.
18. Zeuzem S, et al. Grazoprevir-Elbasvir combination therapy for treatment-naïve cirrhotic and noncirrhotic patients with chronic hepatitis C virus genotype 1, 4 or 6 infection. Ann Int Med 163 (1), 2015, 1-13.
19. Dore GJ, et al.: C-EDGE CO-STAR: efficacy of grazoprevir/elbasvir fixed dose combination for 12 weeks in HCV-infected persons who inject drugs on opioid agonist therapy. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 40
20. Pockros P, et al.: RUBY-I: Ombitasvir/Paritaprevir/Ritonavir + Dasabuvir +/- Ribavirin in Non-irrhotic HCV Genotype 1-infected Patients With Severe Renal Impairment or End-Stage Renal Disease. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 1039
21. Poordad F, et al.: Turquoise-III: 12-week ribavirin-free regimen of ombitasvir/paritaprevir/r and dasabuvir for patients with HCV genotype 1B and cirrhosis. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 1051
22. Zeuzem S, et al.: Predictors of Response to Grazoprevir/Elbasvir Among HCV Genotype 1 (GT1)-Infected Patients: Integrated Analysis of Phase 2-3 Trials. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 700
23. Asselah T, et al. High Efficacy of Grazoprevir and Elbasvir With or Without Ribavirin in 103 Treatment-Naive and Experienced Patients With HCV Genotype 4 Infection: A Pooled Analysis. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 251
24. Jacobson I, et al.: An Integrated Analysis of 402 Compensated Cirrhotic Patients With HCV Genotype (GT) 1, 4 or 6 Infection Treated With Grazoprevir/Elbasvir. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 42
25. Jacobson I, et al.: Prevalence and Impact of Baseline NSA Resistance Associated Variants (RAVs) on the Efficacy of Elbasvir/Grazoprevir (EBR/GZR) Against GT1a Infection. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract LB22
26. Dusheiko G, et al.: Safety and Tolerability of Grazoprevir/Elbasvir in Patients With Chronic Hepatitis C (HCV) Infection: Integrated Analysis of Phase 2-3 Trials. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 712
27. Feld J, et al.: A Phase 3 Double-Blind Placebo-Controlled Evaluation of Sofosbuvir/Velpatasvir Fixed Dose Combination for 12 Weeks in Naïve and Experienced Genotype 1, 2, 4,5, 6 HCV Infected Patients with and without cirrhosis: Results of the ASTRAL-1 Study. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract LB2
28. Feld JJ, et al.: ASTRAL-1 Investigators. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N Engl J Med. 2015 Nov 16. [Epub ahead of print]
29. Sulkowski M, et al.: A Randomized Controlled Trial of Sofosbuvir/GS-5816 Fixed Dose Combination for 12 Weeks Compared to Sofosbuvir with Ribavirin for 12 Weeks in Genotype 2 HCV Infected Patients: The Phase 3 ASTRAL-2 Study. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 205
30. Mangia A, et al.: Sofosbuvir/GS-5816 Fixed Dose Combination for 12 Weeks Compared to Sofosbuvir with Ribavirin for 24 Weeks in Genotype 3 HCV Infected Patients: The Randomized Controlled Phase 3 ASTRAL-3 Study. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 249
31. Charlton M, et al.: Sofosbuvir/Velpatasvir Fixed Dose Combination for the Treatment of HCV in Patients with Decompensated liver Disease: The Phase 3 ASTRAL-4 Study. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract LB13
32. Poordad F, et al.: 98%-100% SVR4 in HCV Genotype 1 Non-Cirrhotic Treatment-Naïve or Pegylated Interferon/Ribavirin Null Responders With the Combination of the Next Generation NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 (SURVEYOR-1). 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 41
33. Wyles DL, et al.: High SVR4 Rates Achieved With the Next Generation NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 in Non-Cirrhotic Treatment-Naïve and Treatment-Experienced Patients With HCV Genotype 2 Infection (SURVEYOR-2). 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 250
34. Kwo P, et al.: High SVR4 Rates Achieved With the Next Generation NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 in Non-Cirrhotic Treatment-Naïve and Treatment-Experienced Patients With HCV Genotype 3 Infection (SURVEYOR-2). 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 248
35. Gane E, et al.: Sofosbuvir/GS-5816+GS-9857 for 6 or 8 Weeks in Genotype 1 or 3 HCV-infected Patients. 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract 38
36. Gane E, et al.: Phase 2, Randomized, Open-Label Clinical Trials of the Efficacy and Safety of Grazoprevir and MK-3682 (NS5B Polymerase Inhibitor) with Either Elbasvir or MK-8408 (NS5A Inhibitor) in Patients with Chronic HCV GT1, 2 or 3 Infection (Part A of C-CREST-1 & 2). 66th Annual Meeting of the American Association for the Study of Liver diseases, November 13-17, 2015, San Francisco, USA; abstract LB15
|
| |
|
 |
 |
|
|