 |
 |
 |
| |
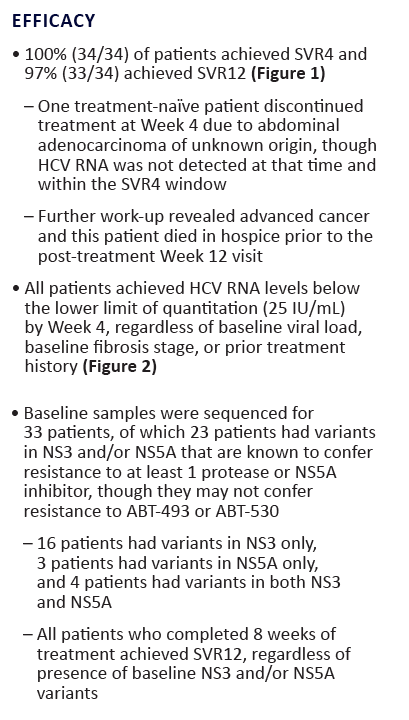
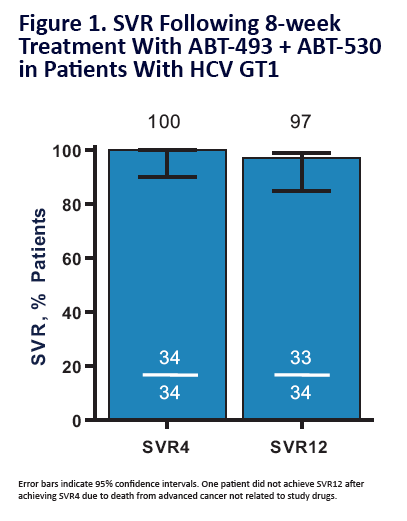
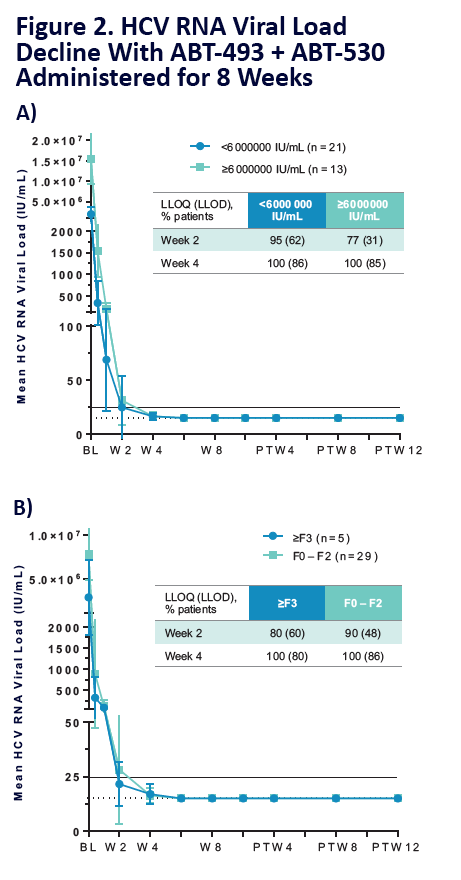
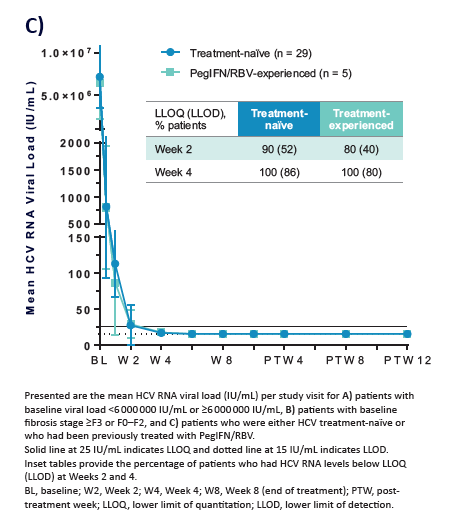
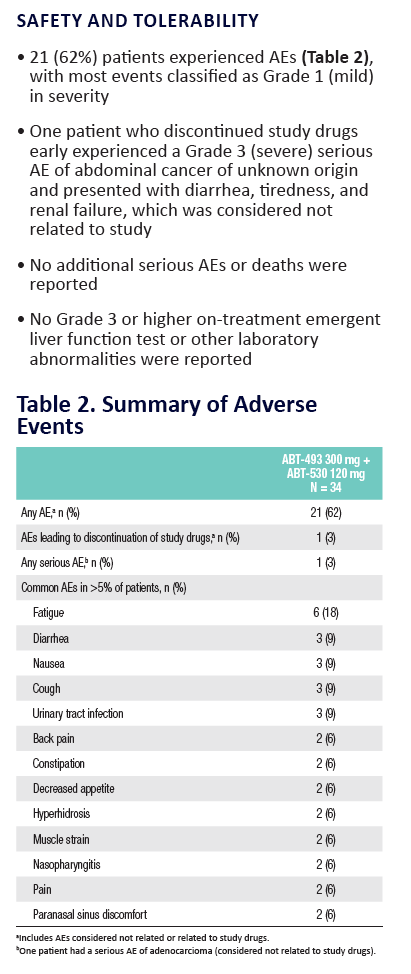
100% SVR4 in HCV Genotype 1 Non-Cirrhotic Treatment-Na´ve or -Experienced Patients With the Combination of ABT-493 and ABT-530 for 8 Weeks (SURVEYOR-I)....[8 weeks]
|
| |
| |
Reported by Jules Levin
AASLD 2015 Nov 13-17 San Francisco
Fred Poordad1, Franco Felizarta2, Armen Asatryan3, Tarek Hassanein4, Humberto Aguilar5, Jacob Lalezari6, J Scott Overcash7, Teresa I Ng3, Ran Liu3, Chih-Wei Lin3, Federico J Mensa3, Jens Kort3
1Texas Liver Insti tute, University of Texas Health Science Center, San Antonio, Texas, United States; 2Private practi ce, Bakersfi eld, California, United States; 3AbbVie Inc., North Chicago, Illinois, United States; 4Southern California GI and Liver Centers and Southern California Research Center,
Coronado, California, United States; 5Louisiana Research Center, Shreveport, Louisiana, United States; 6Quest Clinical Research, San Francisco, California, United States; 7eStudySite, San Diego, California, United States
|
| |
|
 |
 |
|
|