 |
 |
 |
| |
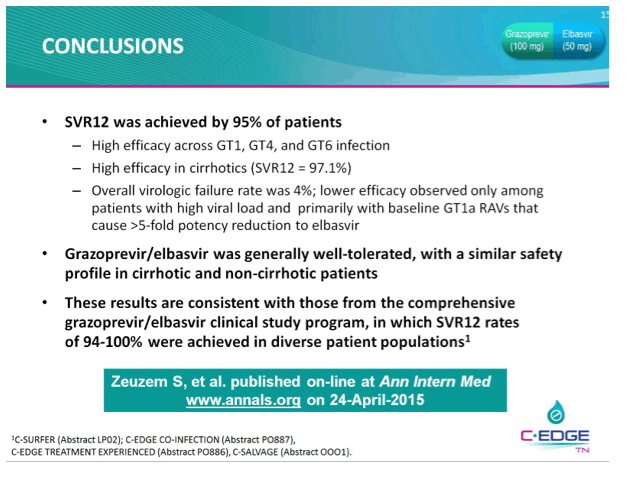
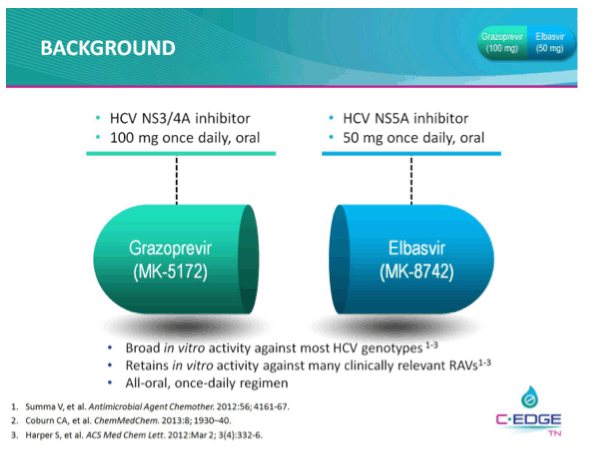
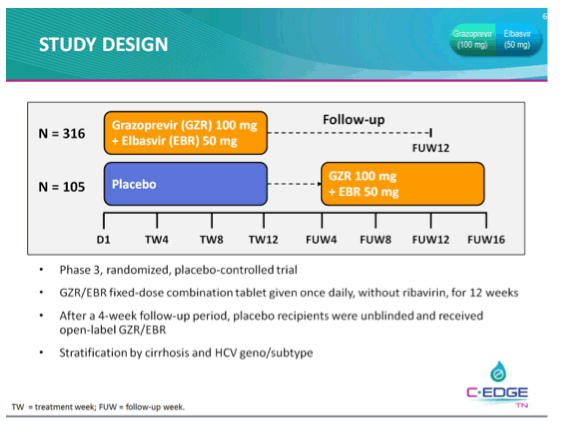
THE PHASE 3 C-EDGE TREATMENT-NAIVE (TN) STUDY OF A 12-WEEK ORAL REGIMEN OF GRAZOPREVIR (GZR, MK-5172)/ELBASVIR (EBR, MK-8742) IN PATIENTS WITH CHRONIC HCV GENOTYPE (GT) 1, 4, OR 6 INFECTION
|
| |
| |
Reported by Jules Levin
EASL 2015 April 22-26 Vienna Austria
Stefan Zeuzem* 1, Reem Ghalib2, K. R. Reddy3, Paul J. Pockros4, Ziv B. Ari5, Yue Zhao6, Deborah Brown7, Mark DiNubile6, Michael Robertson6, Janice Wahl7, Eliav Barr6, Joan Butterton6
1Goethe University Hospital, Frankfurt, Germany, 2Texas Clinical Research Institute, Dallas, 3University of Pennsylvania, Philadelphia, 4Scripps Translational Science Institute, La Jolla, United States, 5Sheba Medical Center, Ramat Gan, Israel, 6Merck, 7Meck, Whitehouse Station, United States

|
| |
|
 |
 |
|
|