| |
Myocardial Infarction, Stroke, and Mortality in cART-Treated HIV Patients on Statins... "Our study did not show a significant benefit of statin therapy on the incidence of MI, stroke, and all-cause mortality in PLWH"
|
| |
| |
Download the PDF here
We evaluated the association of statins with incidence of myocardial infarction (MI), stroke, and all-cause mortality treated as a composite in an HIV infected cohort........Though an extensive literature supports the benefits of statins on survival, cardiovascular outcomes, and lowering of inflammatory biomarkers in HIV-free subjects,18,19 less is known about the benefits of statins in HIV-infected individuals. This topic has recently started to attract the attention of HIV-focused investigator groups. One study showed a mortality benefit far beyond what has been observed in non-HIV infected patients,20 while other studies have failed to show benefits on mortality,21 or mortality and cardiovascular outcomes.22 The latter study,22 however, showed a significant association between statins and a decreased incidence of non-AIDS defining malignancies. No results of randomized trials have been reported so far.
Using data from a prospective cohort of PLWH, we examined the association of statins with the risk of developing MI, stroke, and all-cause mortality......Our primary outcome was the composite endpoint of MI, stroke, or all-cause mortality. Participants' eligible study time continued until the time of the first event within the composite outcome, or until the time of censoring
The average age of our study sample (n=438) was 44 years, 32% were women, and the cohort included 32% black subjects, 10% Hispanic subjects, and 5% subjects did not report their race
The mean follow-up time in subjects who never used statins was 275 weeks (SD=190 weeks). The mean follow up time in statin users was 411 weeks (SD=193 weeks), with the mean accumulated time on statins being 165 weeks (SD=145 weeks).
Our study did not show a significant benefit of statin therapy on the incidence of MI, stroke, and all-cause mortality in PLWH.
Indeed, the only three variables significantly predictive of this outcome were age and smoking (traditional risk factors), as well as CD4 count. Age and CD4 count remained significantly associated with poor outcomes even when our composite endpoint was restricted to MI and stroke only, which is particularly interesting, as CD4 count is not typically considered a cardiovascular risk factor......Our outcome is consistent with some of the prior studies
The time from cART initiation to baseline showed no effect on our composite outcome (HR=1, p=0.72).......We found significant associations between the composite outcome and CD4 count [HR=0.88 (0.83-0.94) per 50 CD4 cells/mL), age (HR=1.07 (1.03-1.1)], and smoking status (HR=1.78 (1.04-3.19)) in both models.
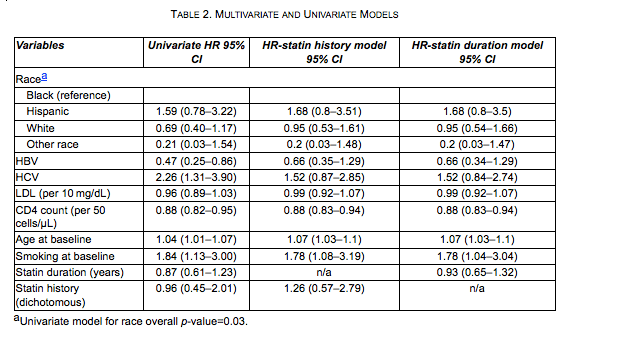
The model results are summarized in Table 2. We were unable to use CRP due to missingness of data (79% at baseline and 75% overall). The HR for statin therapy was 1.26 (0.57-2.79) as a binary variable and 0.93 (0.65-1.32) per year in the statin duration model. To assess the overall benefit of statins, including their lipid lowering effect, we analyzed the same models without adjustment for LDL and there was no change in the statin effect magnitude or its statistical significance: HR=1.26 (0.57-2.78) for positive history of statin use, and HR=0.93 (0.65-1.32) per 1 year of statin use. We found significant associations between the composite outcome and CD4 count [HR=0.88 (0.83-0.94) per 50 CD4 cells/mL), age (HR=1.07 (1.03-1.1)], and smoking status (HR=1.78 (1.04-3.19)) in both models.
In a separate study done in the same cohort, our group demonstrated an association between increased all cause mortality in PLWH, and metabolic syndrome and some of its components, particularly hypertriglyceridemia.27 We have also demonstrated the association between higher cIMT and mortality in this same cohort; cIMT greater than the 75%tile for the cohort were also more likely to die. In this study, CRP was higher in those who died than those who survived. CRP was more likely to be greater than 3 mg/L in those who died.11 These data provided a rationale for this analysis as they suggest that abnormalities in metabolic and vascular health are detrimental to PLWH. Some studies, interestingly, showed that PLWH are in fact at a higher risk of developing type 2 diabetes and that hypertriglyceridemia along with other factors (i.e., age, lower CD4 count) may be independent risk factors.28 The pleiotropic effect of statins involves anti-inflammatory properties, as well as changes in the metabolism of lipids and sugars. These agents are associated with the development of type 2 diabetes and carry an FDA warning for this effect. It is possible that the balance of statin effects in PLWH is less favorable than it is in the general population, making it more neutral overall, even though we hypothesized that the anti-inflammatory property would tip the balance in the other direction.
-----------------
Myocardial Infarction, Stroke, and Mortality in cART-Treated HIV Patients on Statins
AIDS Patient Care and STDs. June 2015
Martin Krsak, MD, MSc,1 David M. Kent, MD, MSc,2,3 Norma Terrin, PhD,3,4 Christina Holcroft, ScD,1 Sally C. Skinner, MA,1 and Christine Wanke, MD1
1Tufts Medical Center and Tufts University, Boston, Massachusetts.
2Predictive Analytics and Comparative Effectiveness (PACE) Center, Tufts Medical Center, Boston, Massachusetts.
3Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, Massachusetts.
4Tufts Clinical and Translational Science Institute, Tufts University, Boston, Massachusetts.
ABSTRACT
Despite combination antiretroviral therapy (cART), people living with HIV (PLWH) continue to have more systemic inflammation and metabolic disturbances than the general population. These risk factors for atherosclerosis and organ dysfunction may be ameliorated by statins. We retrospectively analyzed 438 cART treated PLWH from the Nutrition For Healthy Living (NFHL) cohort to determine the association between statins and myocardial infarction (MI), stroke, and all-cause mortality as a composite. We used Cox proportional hazards regression as our main analysis. The average age was 44 years, 32% were women, and 67 of the 438 subjects used statins. There was no association between statins and our composite endpoint in two separate models [1.26 (0.57-2.79) in statin history model and 0.93 (0.65-1.32) per year in statin duration model]. The composite outcome was significantly associated with CD4 count, age, and smoking status in both models. CD4 count remained significant even after exclusion of mortality from the composite (HR=0.88, p=0.02). Confounding control via propensity scoring and multiple imputations did not change the results. Statins did not have an effect on MI, stroke, and mortality. Interestingly, CD4 count appears to be an important predictor of these outcomes, even after exclusion of death from the composite.
Introduction
HIV in the era of cART has become a chronic disease, and people living with HIV (PLWH) now more frequently die from heart disease, stroke, non-AIDS defining cancers, or organ failure as opposed to AIDS.1 This evolution has been a process in flux since the introduction of AZT as the first therapeutic agent in 1987. HIV, however, did not become the chronic disease we know today until after the introduction of protease inhibitors (PI) in the mid-1990s and their use in combination with nucleoside reverse transcriptase inhibitors. Reduction in morbidity and mortality brought by cART was evident by 2000.2,3 Further evidence of modern cART benefits regarding HIV disease outcomes in the broadest sense has been reviewed in the literature since then.4-6
Despite fully suppressed viral load achieved by modern cART, PLWH have persistently increased systemic inflammation and more pronounced metabolic disturbances compared to the general population.7 Dyslipidemia is a known risk factor for atherosclerosis, and chronic inflammation is an independent risk factor for atherosclerosis8 and neoplasias,9 and can lead to dysfunction in multiple organs.10 Multiple studies have documented increased levels of inflammatory biomarkers [e.g., C-reactive protein (CRP), interleukin-6, sCD14] in HIV patients, as well as their concurrent rise with HIV related disease progression.11-14 3-Hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors (statins), primarily used as serum cholesterol lowering agents, have been shown to suppress inflammation15 by, at present, incompletely understood mechanisms. Clinicians choosing to use statins in HIV patients, however, face challenges beyond complications associated with statin therapy in the general population (e.g., diabetes, myopathy),16 including potential toxicity associated with drug interactions between statins and certain cART agents (particularly PI and NNRTI).17
Though an extensive literature supports the benefits of statins on survival, cardiovascular outcomes, and lowering of inflammatory biomarkers in HIV-free subjects,18,19 less is known about the benefits of statins in HIV-infected individuals. This topic has recently started to attract the attention of HIV-focused investigator groups. One study showed a mortality benefit far beyond what has been observed in non-HIV infected patients,20 while other studies have failed to show benefits on mortality,21 or mortality and cardiovascular outcomes.22 The latter study,22 however, showed a significant association between statins and a decreased incidence of non-AIDS defining malignancies. No results of randomized trials have been reported so far.
Using data from a prospective cohort of PLWH, we examined the association of statins with the risk of developing MI, stroke, and all-cause mortality.
Methods
Cohort description
The Nutrition For Healthy Living (NFHL) cohort was initiated in 1995 to examine the nutritional status and metabolism in a representative cohort of HIV-infected adults from Massachusetts. Since 1995, 881 HIV infected adults have been enrolled on a rolling basis. The NFHL patients were followed for HIV (and its outcomes), other medical conditions, dietary intake, medications, body composition, quality of life, liver function, serum glucose, and insulin levels initially via 6-monthly visits, and later on annually. The exclusion criteria for NFHL included diabetes, uncontrolled hypertension, and myocardial infarction or stroke within the past 6 months. But participants who developed these conditions after enrollment continued in the study and were consented for the CARE sub-study, which focused on cardiovascular health. The CARE subset was begun in 2000 and enrolled any consenting NFHL participants (total n=345). The initiation of this subcohort reflected a new era for the monitoring of HIV-infected patients in general. From September of 2000 on, the participants continued their regular 6-monthly study visits, but the NFHL investigators began collecting data on serum lipid profiles, Framingham risk score, and CRP as well as surrogate markers of cardiovascular disease (carotid intima media thickness (cIMT), and coronary artery calcium (CAC).
Study objectives
We evaluated the association of statins with incidence of myocardial infarction (MI), stroke, and all-cause mortality treated as a composite in an HIV infected cohort.
Inclusion criteria and start of follow up
In our analysis we included only those participants in the NFHL study who at any point (prior to or at baseline) initiated cART (678 subjects). Reflecting the initiation of CARE, the baseline in our study was September 2000 or the date of initiation of cART (whichever occurred later). Lipid level and other cardiovascular parameters were collected on the NFHL participants beyond the CARE subset, and our analyzed group is therefore larger than the CARE subgroup (499 subjects). September 2000 was chosen to address a few important factors: Our analyzed population was selected to represent "modern" HIV patients by both the cART agents used for treatment and by the way they are monitored (not only for HIV but also for cardiovascular and metabolic health). Participants, who reported MI or stroke prior to initiation of cART were excluded, leaving 480 subjects. 41 subjects had no follow up data and one additional subject was excluded based on statin use for a period immediately prior to the baseline but not after, which would have confounded baseline parameters and caused possible misclassification of this subject. We thus identified a total of 438 participants from NFHL cohort for our analysis (Fig. 1).
Outcome definition, censoring criteria, and follow-up time
Our primary outcome was the composite endpoint of MI, stroke, or all-cause mortality. Participants' eligible study time continued until the time of the first event within the composite outcome, or until the time of censoring. Participants were censored at the last known study visit, except that death was followed up for 1 year past the last visit. The period of 1 year was chosen to match the predefined spacing of scheduled visits. cART interruption was not considered as a basis for censoring.
Clinical data
Clinical information was collected at baseline and every 12 months (initially every 6 months). Laboratory data (laboratory methods described elsewhere23) were obtained during the same visit, or as close as possible. Demographic data were assessed via interviewer-administered questionnaires.
Statistical analysis
We chose the Cox proportional hazards method with time varying covariates to evaluate the association of statins with the composite outcome. Besides statin history and duration, other variables used as time varying predictors were LDL cholesterol and CD4 count. Missing values (assumed missing at random, detailed in Table 1) were imputed using Multivariate Imputation by Chained Equations (MICE version 2.18)24 R package, if the missingness was 15% or less. Variables with higher volume of missingness were excluded from further analysis (e.g., CRP). We considered findings from the prior literature and chose baseline predictors based on face validity.
The restriction of baseline to September 2000 or later imposed a potential survivor bias through the inclusion of patients started on cART before 2000. We mitigated this effect by using time from cART initiation to baseline as a continuous variable in the regression model. This variable served as a proxy for the survivor effect as well as for disease duration and stage. Statin use was categorized in two ways: as a dichotomous variable, representing current or prior statin use, which changed from 0 to 1 at the first visit with reported use and remained that way through the end of follow up; we also specified statin use as cumulative, but not necessarily continuously increasing, time on treatment, initiated at the first visit during which the patient reported use. If the treatment was interrupted, the last value was carried forward until statin use was restarted or through the end of follow up. We chose this approach because the main effect of statins is thought to be the prevention of atherosclerotic plaque formation and this effect may stop but is unlikely to be completely reversed after treatment cessation.
We chose to model the two statin specifications separately. One model used statin history, while the other used statin duration as the main predictor of the composite endpoint. The other two time varying predictors, LDL and CD4 count, were specified as their respective continuous values at each follow-up visit. All other predictors carried their baseline value forward. All covariates were analyzed as continuous or dichotomous, depending upon their respective way of reporting in clinical settings. No linear variables were subdivided by cut-points. The choice of predictors for the multivariate models was aided by univariate analyses shown in Table 2.
For multivariate adjustment, we used all statistically significant predictors from the univariate models. We then forced the statin use variable into the model (one at the time, as outlined above), as our main predictor. We also forced LDL levels into the model as a potential important confounder of the main predictor. The main model was applied to the pooled imputed dataset and no automated selection techniques were used to further reduce the number of predictors.
To account for the "healthy user effect" associated with statins, we performed a propensity score adjusted sensitivity analysis of the composite outcome. We calculated two separate sets of propensity scores. For the dichotomously coded history of statin use, the propensity scores for each person-week in follow-up was calculated via a logistic generalized estimating equation (GEE) model, to account for repeated measures within subjects. The propensity score was updated for every unit of analysis (person-time in weeks). The propensity score was then included in the sensitivity analysis as an additional time varying covariate. The predictors selected to predict statin use included: gender, race, age at baseline, HCV and HBV co-infection, presence of metabolic syndrome, Framingham risk score percentage, time varying LDL level, baseline HDL level, CD4 count, time from start of cART, smoking status and baseline protease inhibitor use. A time varying cART use was also added to the propensity score model.
With a GEE model for Poisson distribution (count data), using the same set of predictors, we also calculated a statin duration predicting score to balance our other main predictor of interest (statin duration in years) in another sensitivity analysis. For both statin specifications we performed a simple and a more complex sensitivity analysis. In the simple analysis, we used only the propensity score for the dichotomous determinant of statin use along with statin history as predictors. Similarly, we used statin duration predicting score along with statin duration as the only predictors in this variant of our simple sensitivity analysis. In the more complex analyses, we included all the predictors from the main model along with the propensity score (or the statin duration predicting score) along with the appropriate statin use variable.
In order to evaluate pure cardiovascular outcomes, we analyzed our two main models after excluding all-mortality from our composite endpoint. All of the programming and calculations were performed using R version 3.0.2 "Frisbee sailing" (freely available statistical software).25
Results
Descriptive subject characteristics
The average age of our study sample (n=438) was 44 years, 32% were women, and the cohort included 32% black subjects, 10% Hispanic subjects, and 5% subjects did not report their race. The average duration of cART prior to baseline in the whole analyzed cohort was 2.5 years and all subjects initiated cART at or prior to their respective baseline. 162 (39%) of subjects had an undetectable HIV viral load at baseline. 67 (15%) of the 438 analyzed subjects used statins during follow up. The distribution of all other baseline predictors is listed in Table 1.
The mean follow-up time in subjects who never used statins was 275 weeks (SD=190 weeks). The mean follow up time in statin users was 411 weeks (SD=193 weeks), with the mean accumulated time on statins being 165 weeks (SD=145 weeks). Statin users, therefore, were followed for an average of 246 weeks without being on statin therapy. 141 weeks (SD=190 weeks) of these 246 weeks were analyzed as statin non-use time (prior to initiation of statins), while the remaining 105 weeks were analyzed as statin user time since these occurred during statin therapy interruptions, during which prior statin users were already classified as such (by both statin variables). Six statin users reported statin therapy at their last known follow-up visit and none of them suffered any of the events qualifying for our composite endpoint. These individuals' average follow up was 423 weeks (SD=241 weeks) and only one of these individuals had a short follow up time of 26 weeks. There were 66 outcomes in this dataset. 20 outcomes were due to MI and/or stroke (approximately evenly distributed), and 46 were deaths.
Time on statins
The maximum count of visits during which a subject reported statin use was 11. This occurred in two individuals. Three patients reported statin use during 10 follow-up visits, and additional 12 people reported statin use more than five times. 50 statin users reported five or fewer follow-up periods of statin use. 27 of these subjects (~40% of all statin users) reported statin use on only one or two occasions [15 (22%) and 12 (18%) subjects, respectively].
Univariate and multivariate modeling
The time from cART initiation to baseline showed no effect on our composite outcome (HR=1, p=0.72).
The model results are summarized in Table 2. We were unable to use CRP due to missingness of data (79% at baseline and 75% overall). The HR for statin therapy was 1.26 (0.57-2.79) as a binary variable and 0.93 (0.65-1.32) per year in the statin duration model. To assess the overall benefit of statins, including their lipid lowering effect, we analyzed the same models without adjustment for LDL and there was no change in the statin effect magnitude or its statistical significance: HR=1.26 (0.57-2.78) for positive history of statin use, and HR=0.93 (0.65-1.32) per 1 year of statin use. We found significant associations between the composite outcome and CD4 count [HR=0.88 (0.83-0.94) per 50 CD4 cells/mL), age (HR=1.07 (1.03-1.1)], and smoking status (HR=1.78 (1.04-3.19)) in both models.
Sensitivity analyses with propensity/prediction scores
Our propensity scored sensitivity analyses consistently showed results similar to the main multivariate model (summarized in Table 3).
Sensitivity analysis via evaluation of MI and stroke only as a composite
This reduced the total number of outcomes from 66 to 20. In this setting, only age (8% HR increase for MI and stroke per year, p=0.04) and CD4 count (12% HR reduction of MI and stroke per each additional 50 CD4 cells, p=0.02) remained significantly associated with the outcome. CD4 count maintained the HR even when used alone (p=0.008) or only with either propensity or prediction score (p=0.01), as shown in Table 4.
Discussion
Our study did not show a significant benefit of statin therapy on the incidence of MI, stroke, and all-cause mortality in PLWH. Indeed, the only three variables significantly predictive of this outcome were age and smoking (traditional risk factors), as well as CD4 count. Age and CD4 count remained significantly associated with poor outcomes even when our composite endpoint was restricted to MI and stroke only, which is particularly interesting, as CD4 count is not typically considered a cardiovascular risk factor. It is highly unlikely that these factors represent the totality of important prognostic factors. Larger studies will be needed to find additional important factors, and to further clarify the possible role of statins in averting this outcome. Given the clinical importance of non-AIDS outcomes for PLWH receiving modern cART, and the fact that primary prevention studies from non-HIV infected patients are unlikely to generalize to this medically complex population, the role of statins should be clarified with an experimental (randomized) study, such as the NIH funded REPRIEVE (A5332) trial,26 which will be starting shortly at 100 U.S. clinical sites and aims to assess 6500 patient randomized to pitavastatin or placebo and will monitor patients for outcomes similar to ours. Furthermore, this trial is specifically designed to evaluate both the metabolic and the inflammatory pathway changes leading up to cardiovascular disease in HIV-positive patients.
Our outcome is consistent with some of the prior studies.21,22 In our study design and statistical approach, we focused on correcting the issues that would have created significant noise or bias in this complex analysis (multiple imputation of missing data; propensity scored sensitivity analyses to account for confounding by indication for statins; statistical adjustment for less than ideal baseline, imposed by the evolution of clinical practice). We also significantly benefited from working with a prospectively collected dataset from a cohort longitudinally monitored for numerous parameters. The wide range of monitored parameters focused not only on HIV and its complications but also on cardiovascular health, nutritional status, and other more general health-related and social factors that are essential for a complex analysis such as this one.
Our study had several limitations. Although the cohort data was prospectively collected, our study was observational. We would have included other hard clinical outcomes in our composite (e.g., neoplasias) but were unable to do so reliably. We were also unable to look at the association between the statin effect and the outcome via comparing unadjusted versus CRP or other inflammatory marker adjusted analyses. This was due to the volume of missing data that was simply too great and prohibitive of imputation.
There were several measurement imprecisions in the calculation of the time under statins. Not knowing the exact time of statin initiation resulted in choosing the time of earliest report to be time zero. This approach was conservative as it was almost certain to start the count later than it actually occurred, such time underestimation may bias the results in slight overestimation of the effect per week of statin use. This would be presumably balanced by overestimating the time at the tail end of use, as once statin was reported it was counted as the whole period. This was designed specifically to balance the potential for underestimation at the beginning and overestimation at the end, respectively.
These measurement imprecisions were the most problematic in users who report use in only one period (15 subjects, 22% of users). We, however, adapted the intention to treat analysis approach and counted these subjects as statin users.
We believe that statins prevent cardiovascular outcomes by primarily preventing the slow build up of arterial plaque, while the role of immediate level of inflammation at the time of a cardiovascular event is less well understood. We had to deal with two major opposing risks when deciding on how to handle statin treatment interruptions. There was a risk of discounting the accrued effect of statins on plaque build-up in individuals previously on statins, if individuals with history of statin use were analyzed as non-users. On the other hand, there was a less well-defined risk of false attribution of statin effect to people with statin use history during a period of interruption. We found it more appropriate to count previous users as always users in the dichotomous statin history analysis. To refine this relatively crude statistical definition, we developed our statin duration model, which allowed us to stop the count of weeks during the interruptions. We decided on this approach understanding that, under these conditions, our analysis would be fundamentally wrong only if the most important protective (or harmful) factor was a physiological effect that requires immediate presence of statins in the organism, which is a much riskier assumption as it is unlikely that such factor would have a role in the lipid plaque build-up process. In the inflammatory pathway, it is even less clear how much of the problem is attributable to the slow histological changes within the cardiovascular system due to chronic inflammation, and how much of a role the immediate level of inflammation would play. In HIV patients, the use of statins is further complicated by potential drug-drug interactions with cART (mainly with PI). This may also mean that the potential benefits are counterbalanced by additional toxicity or interactions that may lower serum levels of cART or statins.
In a separate study done in the same cohort, our group demonstrated an association between increased all cause mortality in PLWH, and metabolic syndrome and some of its components, particularly hypertriglyceridemia.27 We have also demonstrated the association between higher cIMT and mortality in this same cohort; cIMT greater than the 75%tile for the cohort were also more likely to die. In this study, CRP was higher in those who died than those who survived. CRP was more likely to be greater than 3 mg/L in those who died.11 These data provided a rationale for this analysis as they suggest that abnormalities in metabolic and vascular health are detrimental to PLWH. Some studies, interestingly, showed that PLWH are in fact at a higher risk of developing type 2 diabetes and that hypertriglyceridemia along with other factors (i.e., age, lower CD4 count) may be independent risk factors.28 The pleiotropic effect of statins involves anti-inflammatory properties, as well as changes in the metabolism of lipids and sugars. These agents are associated with the development of type 2 diabetes and carry an FDA warning for this effect. It is possible that the balance of statin effects in PLWH is less favorable than it is in the general population, making it more neutral overall, even though we hypothesized that the anti-inflammatory property would tip the balance in the other direction.
In summary, our small observational study did not show significant association between statins and the composite outcome of MI, stroke, or all-cause mortality. It did, however, suggest that CD4 count preservation might play a protective role not only for the whole composite but also for pure cardiovascular outcomes after excluding death as an outcome from the analysis.
A larger prospective trial, such REPRIEVE26 scheduled to start in 2015, could further clarify the benefits and harms in this population, if it includes monitoring patients for metabolic and inflammatory parameters, multiple hard clinical outcomes, as well as for medication side effects and drug. Softer clinical outcomes leading up to MI, stroke, and mortality (such as increasing cIMT or CAC) could also be considered as a target for analysis.
The importance of CD4 count in our study deserves further confirmation in research, as the implications would include yet another argument supporting earlier cART initiation.
|
|
| |
| |
|
|
|