| |
Long-term mortality in HIV-infected individuals 50 years or older:
a nationwide, population-based cohort study (Denmark)
|
| |
| |
Download the PDF here
Mortality in HIV vs HIV-neg Group in Denmark with & Without Comorbidities for HIV+ >50 years old.....[from Jules: of note HIV+ have more comorbidities vs HIV-neg CCI index is double in HIV+ vs HIV-neg.....study authors conclude: We detected a 1.6-fold increased risk of death of among HIV-infected individuals ≥ 50 years without comorbidity compared to population controls without comorbidity.....also of note since this study was conducted in Denmark it could not consider racial or ethnic differences we have in the US regarding HIV treatment & prevalence of comorbidities; in this study 88% of the patients were white, only 7% were IDUs-a different patient population than in the USA; its also a different healthcare system & culture in Denmark than in the USA]
"Our aim was to estimate long-term mortality among HIV-infected individuals who were 50 years or older, when compared to an individually-matched cohort from the background population."
"We conclude that survival after age 50 has improved markedly in the HIV-population within the cART-era, but is still substantially lower than in the background population. Even among well treated HIV-infected individuals ≥ 50 years without comorbidity or AIDS-defining events estimated median survival time remained considerably lower than in the background population. Our results indicate that further incentives to reduce mortality among HIV-infected individuals ≥ 50 years are needed.
To our knowledge, this is the first nationwide study to estimate long-term mortality among 50-80 year old HIV-infected individuals compared to an individually-matched cohort from the background population.
In general HIV-infected individuals are known to have an increased mortality compared to the general population [21].
In our study the proportion of HIV-infected individuals ≥ 50 years with CCI ≥ 1 (number of comorbidities, see below for list of conorbidities including HCV, IDU) at study inclusion was higher than among the age-matched population controls. Lohse et al. has previously shown that comorbidity achieved prior to HIV-diagnosis substantially influences mortality following HIV diagnosis, and that HIV-infection and comorbidity interact synergistically with the risk of death among HIV-infected individuals [24]. In our study we included a well-treated subpopulation of HIV infected individuals ≥ 50 years with undetectable HIV RNA and CD4 cell count ≥ 350 cells/μl after year of cART. We detected a 1.6-fold increased risk of death of among HIV-infected individuals ≥ 50 years without comorbidity compared to population controls without comorbidity, which is in line with the findings of Lohse et al. who found a 1.7-fold increased mortality in HIV-infected individuals with CCI=0 compared to population controls with CCI=0.
Ageing of the HIV-population emphasizes challenges in HIV-treatment, as HIV-infected individuals ≥ 50 years more often faces complicating factors such as higher prevalence of comorbidity, more advanced HIV-infection at time of diagnosis 1 and polypharmacy [4,5]. In current Danish HIV treatment guidelines age ≥ 50 years is a relative indication for early start of cART irrespective of CD4 cell count [23].
Among HIV-infected individuals estimated median survival time increased during the study period; 11.8 years (95 % CI; 10.2-14.5) in 1996-1999, 17.8 years (95 % CI; 15.1-20.5) in 2000-2005, and 22.5 years (95 % CI; 20.0-24.2) in 2006-2014. For population controls estimated median survival time was 30.2 years (95% CI; 29.5-31.1) in 1996-2014. MR, EMR and MRR in 5-year age-intervals for HIV-infected individuals and population controls are summarized in table 2.
Among HIV-infected individuals without comorbidity the estimated median survival time from age 50 years was 25.6 years (to age 75.6 years) (95% CI; 23.8-NA) compared to 34.2 years (age 84.2 years) (95% CI; 29.6-38.3) among population controls without comorbidity. MRR was 1.7 (95%; 1.2-2.3) for HIV-infected individuals compared to population controls."
MR= all-cause mortality risk; excess mortality rates (EMR), and mortality rate ratios (MRR)
Long-term mortality in HIV-infected individuals 50 years or older: a nationwide, population-based cohort study.
JAIDS Aug 27 2015
Legarth, Rebecca; Ahlstrom, Magnus G; Kronborg, Gitte; Larsen, Carsten S.; Pedersen, Court; Pedersen, Gitte; Mohey, Rajesh; Gerstoft, Jan; Obel, Niels
1Department of Infectious Diseases, Copenhagen University Hospital, Rigshospitalet, Denmark.
2Department of Infectious Diseases, Copenhagen University Hospital, Hvidovre, Denmark.
3Department of Infectious Diseases, Aarhus University Hospital, Denmark.
4Department of Infectious Diseases, Odense University Hospital, Denmark.
5Department of Infectious Diseases, Aalborg University Hospital, Denmark.
6Department of Internal Medicine, The Regional Hospital West Jutland, Denmark.
Abstract
Background: Although the prevalence of HIV-infection among individuals >= 50 years of age has increased, the impact of HIV-infection on risk of death in this population remains to be established. Our aim was to estimate long-term mortality among HIV-infected individuals who were 50 years or older, when compared to an individually-matched cohort from the background population.
Methods: Population-based cohort-study including HIV-infected individuals >= 50 years, who were alive one year after HIV-diagnosis (n=2,440) and a comparison cohort individually-matched by age and gender extracted from the background population (n=14,588). Cumulative survival was evaluated using Kaplan-Meier method and Mortality Rate Ratios (MRRs) were estimated using Cox Regression Models. Study period 1996-2014.
Results: Estimated median survival time from age 50 years for HIV-infected individuals increased from 11.8 years (95% CI; 10.2-14.5) during 1996-1999 to 22.8 years (20.0-24.2) in 2006-2014. MRR decreased with increasing age from 3.8 (3.1-4.7) for 50-55 years to 1.6 (1.0-2.6) for 75-80 years. In a cohort of well-treated HIV-infected individuals >= 50 years without AIDS-defining events or comorbidity at study inclusion (n=517). MRR was 1.7 (1.2-2.3) compared to population controls without comorbidity.
Conclusion: Among HIV-infected individuals estimated median survival time from age 50 years has increased by more than 10 years from 1996-1999 to 2006-2014, but is still substantially lower than in the background population. Even among well-treated HIV-infected individuals >= 50 years without comorbidity or AIDS-defining events the estimated median survival time remains lower than in the general population.
Introduction
In high-income countries approximately 30-50 % of HIV-infected individuals are 50 years or older, and the prevalence of HIV-infection among individuals older than 50 years of age is increasing regardless of geographical regions [1,2].
Ageing of the HIV-population is the result of the tremendous decline in mortality following the introduction of combination antiretroviral therapy (cART) in the mid-1990 [3], and an increased proportion of newly HIV-infected individuals diagnosed after 50 years of age [4-6]. HIV-infection in older individuals is often associated with several complicating factors such as higher prevalence of comorbidity, higher risk of late HIV11 diagnosis, increased mortality [6-12] and reduced CD4-cell response to cART [13-15]. With the increased prevalence of HIV-infections among older individuals it has become important to determine, whether the effect of cART among older HIV-infected individuals are reflected by mortality rates approaching those in the background population.
We used a nationwide, population based cohort of HIV-infected individuals 50 years or older and an individually-matched comparison cohort from the background population to estimate all-cause mortality stratified by age-intervals and calendar period. We further compared mortality among well-treated HIV-infected individuals ≥ 50 years without comorbidity with an individually-matched comparison cohort from the background population also without comorbidity.
Methods
Setting
The study period was from 1 January 1996 until 31 May 2014. The estimated HIV prevalence in Denmark is approximately 0.1 % in the adult population, and HIV-treatment is centralized to eight specialized HIV-centers where HIV-infected individuals are seen on an outpatient basis at intended intervals of 12 - 24 weeks. The Danish Healthcare system provides free tax-supported HIV6 treatment including cART to all HIV-infected individuals that are residents in Denmark.
Data sources
The Danish HIV Cohort Study (DHCS)
DHCS is a population-based, nationwide cohort study including all HIV-infected individuals treated at Danish HIV centers since 1 January 1995. Individuals are consecutively enrolled at first contact with an HIV center. Collected data includes demographics, date of HIV diagnosis, route of transmission, AIDS-defining events, and antiretroviral treatment. CD4 cell counts and HIV RNA measurements are extracted electronically from laboratory data files. Data are updated annually [16].
We used the unique 10-digit civil registration number assigned to all Danish residents to link the following registries:
Civil Registration System (CRS):
CRS includes information on vital status, date of death, and migration for all Danish residents. Data is updated continuously [17].
The Danish National Registry of 1 Patients (DNRP):
DNRP includes information on all patients discharged from Danish hospitals. Records for each in patient admission include dates of admission and discharge and diagnoses, categorized according to the International Classification of Diseases (ICD-8/ICD-10) [18].
Study populations
All HIV-1 infected individuals who reached an age of 50 years between 1 January 1996 and 31 May 2014 were included in study. Study inclusion was one year following HIV-diagnosis, one year following immigration, or date of 50th birthday, whichever came last. For each HIV-infected individual six population controls individually matched on age and gender were identified from CRS to constitute a background population cohort. All population controls were living in Denmark at time of study inclusion of their corresponding HIV-infected individual. Population controls were assigned same date of study inclusion as their corresponding HIV-infected individual.
HIV-infected individuals and population controls were categorised with comorbidity at study inclusion if they: had Charlson Comorbidity Index (CCI) ≥ 1 point, were diagnosed with hepatitis C virus- (HCV) in DNRP or positive HCV antibody test or a positive HCV RNA test in DHCS, diagnosed with alcohol- or drug-related abuse in DNPR, or were registered as intravenous drug abusers (IDU) in DHCS.
CCI was calculated at date of study inclusion based on recordings in DNRP, and includes 19 specific diseases: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, connective tissue disease, ulcer disease, mild liver disease, diabetes, hemiplegia, moderate to severe renal disease, diabetes with end-organ failure, any tumor, leukemia, lymphoma, moderate to severe liver disease, metastatic solid tumor. AIDS was not included. CCI was calculated by methods described previously [19].
Further, from the study population we identified a cohort of well-treated HIV-infected individuals observed during 2006-2014 who: 1) had received at least 1 year of cART, 2) had HIV RNA < 500 copies/ml and CD4 cell count ≥ 350 cells/μl after 1 year of cART, 3) had no AIDS-defining events at study inclusion or after 1 year of cART, and 4) had no comorbidity (as defined below) after 1 year of cART. Study inclusion was one year after start of cART.
For each well-treated HIV-infected individual six population controls individually matched on age and gender were identified in CRS. Population controls had to be living in Denmark at time of study inclusion of their corresponding HIV-infected individual, and be without any record of comorbidity at the time of study inclusion. Population controls were assigned the same date of study inclusion as their corresponding HIV-infected individual.
All-cause mortality rates (MR) per 1,000 Person Years at Risk (PYR), excess mortality rates (EMR) per 1,000 PYR and mortality rate ratios (MRR) for 5-years age intervals for HIV-infected individuals and population controls were calculated for the complete study period and the calendar periods: 1996-1999, 2000-2005 and 2006-2014. MRRs were estimated using Cox Proportional Hazards Model. The proportional-hazards assumption was tested using non-zero slope.
RESULTS
We identified 2,440 HIV-infected individuals, who fulfilled the inclusion criteria and who were followed for a total of 16,637 PYR (Table I). Among HIV-infected individuals 530 (21.7 %) died, 50 (2.1 %) emigrated and 4 (0.2 %) were registered as lost to follow up. We identified 14,588 population controls that were followed for a total of 115,817 PYR. Among population controls 1,388 (9.5 %) died, 185 (1.3 %) emigrated and 8 (0.1 %) were recorded lost to 1 follow up. Further baseline characteristic are summarized in table 1.
All-cause mortality among HIV-infected individuals compared to population controls aged 50
years or older.
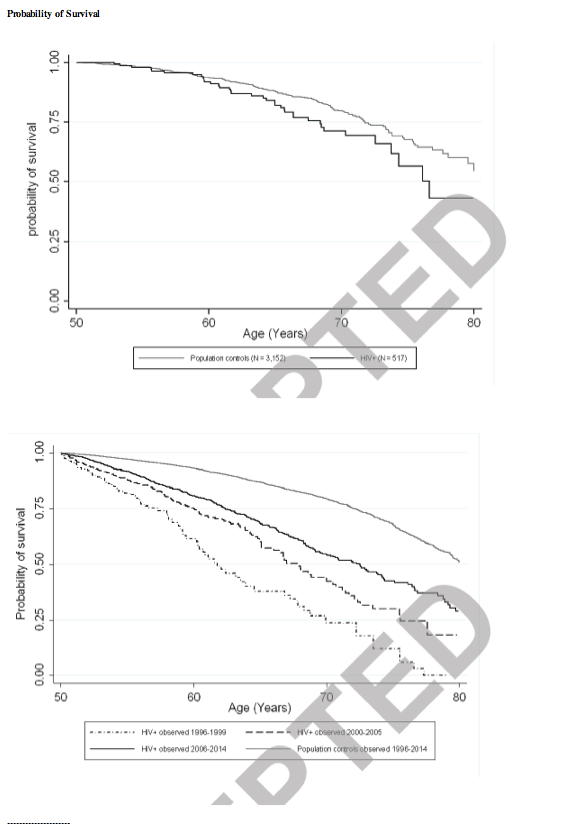
Figure 1 shows Kaplan-Meier survival curves from 50 years of age for HIV-infected individuals and population controls stratified by calendar period of 1996-1999, 2000-2005 and 2006-2014.
Among HIV-infected individuals estimated median survival time increased during the study period; 11.8 years (95 % CI; 10.2-14.5) in 1996-1999, 17.8 years (95 % CI; 15.1-20.5) in 2000-2005, and 22.5 years (95 % CI; 20.0-24.2) in 2006-2014. For population controls estimated median survival time was 30.2 years (95% CI; 29.5-31.1) in 1996-2014. MR, EMR and MRR in 5-year age-intervals for HIV-infected individuals and population controls are summarized in table 2.
The overall-MRR decreased with increasing age; from 3.8 (95% CI; 3.1-4.7) for 50-55 years to 1.6 (95% CI; 1.0-2.6) for 75-80 years of age (table 2). The overall-EMR increased with increasing age; from 16.3 (95% CI; 12.6-19.9) for 50-55 years to 48.8 (95% CI; 7.2-90.4) for 75-80 years (table 2). Highest EMR was observed during the period 1996-1999, and remained lower within all age intervals in the two subsequent periods (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A739.). In line with this, MRRs were highest in the early period 1996-1999, and remained lower in all age-intervals in the two subsequent periods 1 (see Table S1, Supplemental Digital Content, http://links.lww.com/QAI/A739).
HIV-infected individuals who were observed during 1996-1999 had increased mortality within all age-strata compared to HIV-infected individuals observed during 2006-2014: : MRR 1.5 (95 % CI; 1.0-2.4) for 50-55 years, 1.8 (95% CI; 1.0-3.1) for 55-60 years, 2.5 (95% CI; 1.3-4.7) for 60-65 years, 1.2 (95% CI; 0.5-3.0) for 65-70 years, 1.2 (95% CI; 0.3-5.5) for 70-75 years and 9.5 (95% CI; 1.7 53.7) for 75-80 years. For HIV-infected individuals observed during 2000-2005 MRRs were 1.5 (95% CI; 1.0-2.2) for 50-55 years, 1.2 (95% CI; 0.8-1.8) for 55-60 years, 1.3 (95% CI; 0.8-2.0) for 60-65 10 years, 1.6 (95% CI; 0.9-2.8) for 65-70 years, 1.9 (95% CI; 0.0-3.9) for 70-75 years and 1.8 (95% CI; 0.5-6.3) for 75-80 years compared to HIV-infected individuals observed during 2006-2014.
To determine whether age at time of HIV diagnosis influenced risk of death, we compared mortality in patients diagnosed prior to or after 50 years for all calendar periods, and observed no substantial difference: MRR 1.0 ( 95 % CI; 0.5-1.9) for 1996-1999, 1.2 (95 % CI; 0.8-1.8) for 2000- 2005 and 1.0 (95 % CI; 0.8-1.4)for 2006-2014.
Mortality among well-treated HIV-infected individuals > 50 years observed during 2006-2014.
We included 517 well-treated HIV-infected individuals without AIDS-defining events or comorbidity at time of study inclusion. Median age at HIV diagnosis vas 45 (IQR; 39-52) and 17 (32.9 %) were older than 50 years of age when diagnosed. Further baseline characteristics for the cohort of well-treated HIV-infected individuals are summarized in table 1. In addition, 3,192 individually-matched population controls without comorbidity were included.
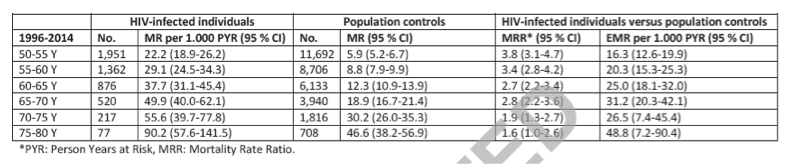
Figure 2 shows Kaplan-Meier survival curves for HIV-infected individuals ≥ 50 years without comorbidity and population controls ≥ 50 years without comorbidity for the period 2006-2014.
Among HIV-infected individuals without comorbidity the estimated median survival time from age 50 years was 25.6 years (to age 75.6 years) (95% CI; 23.8-NA) compared to 34.2 years (age 84.2 years) (95% CI; 29.6-38.3) among population controls without comorbidity. MRR was 1.7 (95%; 1.2-2.3) for HIV-infected individuals compared to population controls.
Discussion
In this population-based study we found that mortality among HIV-infected individuals ≥ 50 years decreased markedly during the period 1996-2014. During the study period mortality decreased within all age strata between 50-80 years of age, but estimated median survival time from age 50 years still remained substantially lower than in the background population. We found no differences in mortality when analyses were stratified by HIV-diagnosis prior to or after 50 years of age. Even among well-treated HIV-infected individuals ≥ 50 years without AIDS-defining events or comorbidity the estimated median survival time was still markedly lower than in the background population.
To our knowledge, this is the first nationwide study to estimate long-term mortality among 50-80 year old HIV-infected individuals compared to an individually-matched cohort from the background population. Lewden et al. found that standardized mortality ratios (SMR) for well-treated HIV-infected individuals with CD4 cell count ≥ 500 cells/mm3 decreased with increasing age from 8.5 for < 40 years to 1.7 for ≥ 60 years [10]. Our findings are in line with these findings, as we also detected decreasing MRRs with increasing age. Within the younger age-intervals we found MRR to be at a lower level than SMRs found by Lewden et al., which could be explained by the lower proportion of IDUs included in our study population (7% vs. 16 %). Further, we excluded HIV-infected individuals who died within the first year following HIV-diagnosis. Within the older age-intervals we found MRR to be slightly higher than SMRs found by Lewden et al., which could rely on the fact that we compare mortality within an HIV-population regardless of CD4 cell count with the general population. When only including HIV-infected individuals with HIV RNA ≤ 500 copies/ml and CD4 cell count ≥ 350 cells/μl following 1 year of cART MRR was in line with the findings of Lewden et al. In general HIV-infected individuals are known to have an increased mortality compared to the general population [21]. However, Obel et al. have found a MRR of 1.14 (95% CI; 0.58-2.23) among HIV-infected individuals aged 45-65 years without HIV risk factors, comorbidity or abuse compared to a cohort of population controls also without comorbidity or abuse [22].
We found that during the study period the median estimated survival time for an HIV-infected individual from age 50 increased by more than 10 years, and these findings supports previous findings by Lohse et al. in a younger HIV-population [3].
The prevalence of HIV-infection is increasing within individuals ≥ 50 years due to improved survival among HIV-infected individuals combined with an increasing number of new HIV-diagnoses among individuals ≥ 50 years [1,2]. Ageing of the HIV-population emphasizes challenges in HIV-treatment, as HIV-infected individuals ≥ 50 years more often faces complicating factors such as higher prevalence of comorbidity, more advanced HIV-infection at time of diagnosis 1 and polypharmacy [4,5]. In current Danish HIV treatment guidelines age ≥ 50 years is a relative indication for early start of cART irrespective of CD4 cell count [23].
In our study the proportion of HIV-infected individuals ≥ 50 years with CCI ≥ 1 at study inclusion was higher than among the age-matched population controls. Lohse et al. has previously shown that comorbidity achieved prior to HIV-diagnosis substantially influences mortality following HIV diagnosis, and that HIV-infection and comorbidity interact synergistically with the risk of death among HIV-infected individuals [24]. In our study we included a well-treated subpopulation of HIV infected individuals ≥ 50 years with undetectable HIV RNA and CD4 cell count ≥ 350 cells/μl after year of cART. We detected a 1.6-fold increased risk of death of among HIV-infected individuals ≥ 50 years without comorbidity compared to population controls without comorbidity, which is in line with the findings of Lohse et al. who found a 1.7-fold increased mortality in HIV-infected individuals with CCI=0 compared to population controls with CCI=0.
Mortality is markedly increased in the first year following HIV-diagnosis [25-27]. To exclude this "lead in" mortality, we only included HIV-infected individuals who were alive one year following HIV diagnosis. As HIV-infected individuals who died within the first year of HIV-diagnosis are more likely to have advanced HIV-infection at time of diagnosis, the study population included in this study will not be representative for an unselected HIV-population ≥ 50 years. On the same basis, prevalence of comorbidity among HIV-infected individuals ≥ 50 years are also likely to be underrepresented in our study compared to an unselected HIV-population ≥ 50 years.
Major strength of our study is the nationwide and population-based design in 1 combination with long and nearly complete follow-up. The population-based design minimizes risk of selection and referral biases. Furthermore, access to Danish registries allowed us to identify a well-matched comparison cohort from the background population.
Our study relied on register-based data and can therefore be subject to misclassification. As we obtained data on outcome variables from the same registers we presume, that a potential misclassification in outcome (death) is non-differential and thereby has minimal influence on our estimates of relative risk. In our study, the definition of comorbidity was based on Charlson comorbidity index score (CCI). CCI was estimated by extracting ICD-10 codes from the Danish National Patient Registry, which includes information on all patients discharged from all Danish hospitals. However, diseases not registered in the Danish National Patient Registry or diseases not included in the CCI will be missed in the calculation of CCI. Further, numbers of events and person years at risk were limited in the very old age-intervals.
We conclude that survival after age 50 has improved markedly in the HIV-population within the cART-era, but is still substantially lower than in the background population. Even among well treated HIV-infected individuals ≥ 50 years without comorbidity or AIDS-defining events estimated median survival time remained considerably lower than in the background population. Our results indicate that further incentives to reduce mortality among HIV-infected individuals ≥ 50 years are needed.
|
|
| |
| |
|
|
|