 |
 |
 |
| |
Lack of Drug Interactions between Boosted and Unboosted Tenofovir Alafenamide-based Antiretroviral Single Tablet Regimens (Emtricitabine/Rilpivirine/Tenofovir Alafenamide and Elvitegravir/Cobicistat/Emtricitabine/Tenofovir Alafenamide) and the anti-HCV Single Tablet Regimen Ledipasvir/Sofosbuvir...... "R/F/TAF or E/C/F/TAF may be coadministered with LDV/SOF without dose modification."
|
| |
| |
Reported by Jules Levin
IDSA 2015, October 7-11, 2015, San Diego, CA
Joseph Custodio, PhD, Eileen Doyle, PharmD, Phillip S. Pang, MD, PhD, Moupali Das, MD, MPH, Huyen Cao, MD, Grace Ma, PhD, Anita Mathias, PhD and Julia Zack, PharmD, Gilead Sciences, Inc., Foster City, CA
Background:
Almost one third of HIV-infected individuals are co-infected with hepatitis C virus (HCV). Use of anti-HCV agents such as ledipasvir (LDV), a P-glycoprotein (Pgp) inhibitor, with HIV antiretrovirals (ARV) such as tenofovir alafenamide (TAF), a Pgp substrate, may be complicated by drug-drug interactions (DDIs). Two Phase 1 studies evaluated DDIs between TAF-based regimens rilpivirine (RPV; R)/emtricitabine (FTC; F)/TAF or elvitegravir/cobicistat/ FTC/TAF (E/C/F/TAF) and the fixed-dose combination anti-HCV LDV/sofusbuvir (SOF).
Methods:
In two multiple-dose, randomized, crossover studies, healthy volunteers received R/F/TAF (25/200/25 mg) [Study 1] or E/C/F/TAF (150/150/200/10 mg) [Study 2]), alone or in combination with LDV/SOF, daily with food for 11 [Study 1] or 10 [Study 2] days.
Plasma concentrations of RPV, EVG, COBI, FTC, TAF and TFV (TAF metabolite), LDV, SOF and GS-331007 (SOF metabolite) were analyzed and PK parameters calculated via noncompartmental analysis. Geometric least squares mean ratio (GMR; combination vs. alone) and 90% confidence intervals (CI) for analytes' AUC, Cmax and Ctau were estimated by linear mixed effect modeling and compared to lack of alteration bounds (70-143% except RPV: 80-125%). Safety was assessed throughout the studies.
Results:
Forty of 42 subjects (Study 1) and 30/30 subjects (Study 2) completed study. Treatments were generally well tolerated.
The GMR and 90% CI for all analytes' PK parameters are:
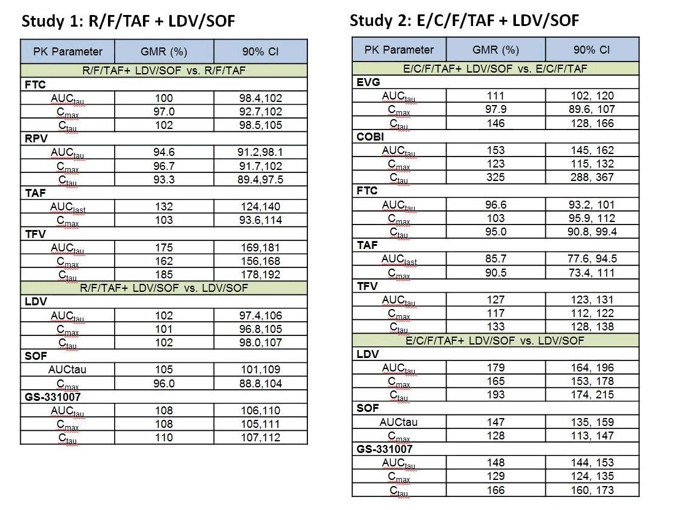
GLSM and 90% CIs for all analytes were contained within the prespecified bounds except TFV when RPV/FTC/TAF was administered with LDV/SOF, COBI, when E/C/F/TAF was coadministered with LDV/SOV, and LDV and SOF when administered with E/C/F/TAF.
Conclusion:
Despite increase in TFV exposure upon coadministration of R/F/TAF + LDV/SOF, the mean TFV AUCtau is ~ 5 times lower than TFV from TDF, and within the range of TFV that did not lead to renal AEs or bone loss (E/C/F/TAF Phase 3). There is no association between higher COBI exposure and incidence of AEs, renal function parameters (E/C/F/TAF Phase 2/3 data). Exposures of LDV and SOF, while higher, are in the exposure-safety range (data from LDV/SOF development program).
R/F/TAF or E/C/F/TAF may be coadministered with LDV/SOF without dose modification.
|
| |
|
 |
 |
|
|