 |
 |
 |
| |
Doubling Dolutegravir Dose Has Little Impact on Controlling Resistant HIV
|
| |
| |
16th International Workshop on Clinical Pharmacology
of HIV and Hepatitis Therapy
May 26-28, 2015
Washington, DC
Mark Mascolini
Logistic regression modeling indicated that raising the dolutegravir dose from 50 to 100 mg twice daily would have little impact on 24-week virologic response of integrase inhibitor-resistant virus [1]. The model also figured that taking dolutegravir with strong enzyme inducers only slightly lowered 24-week response of resistant virus.
Based on results of the VIKING, VIKING-3, and VIKING-4 trials, dolutegravir is licensed at a dose of 50 mg twice daily for (1) people with certain integrase inhibitor resistance mutations and (2) treatment-naive or experienced people also taking a potent UGT1A or CYP3A inducer (efavirenz, fosamprenavir/ritonavir, tipranavir/ritonavir, or rifampin). Product information notes that dolutegravir efficacy wanes in patients with a mutation at integrase position Q148 plus two or more other integrase mutations. (Dolutegravir is also licensed at 50 mg once daily for people who have not taken an integrase inhibitor.)
This modeling study aimed to determine the dolutegravir exposure-response relationship at 24 weeks in people with integrase inhibitor resistance mutations and taking dolutegravir with (1) food (which increases dolutegravir exposure), (2) enzyme-inducing drugs (which lower dolutegravir exposure), or (3) metal cation-containing dietary supplements, such as multivitamins with iron or zinc (which lower dolutegravir exposure).
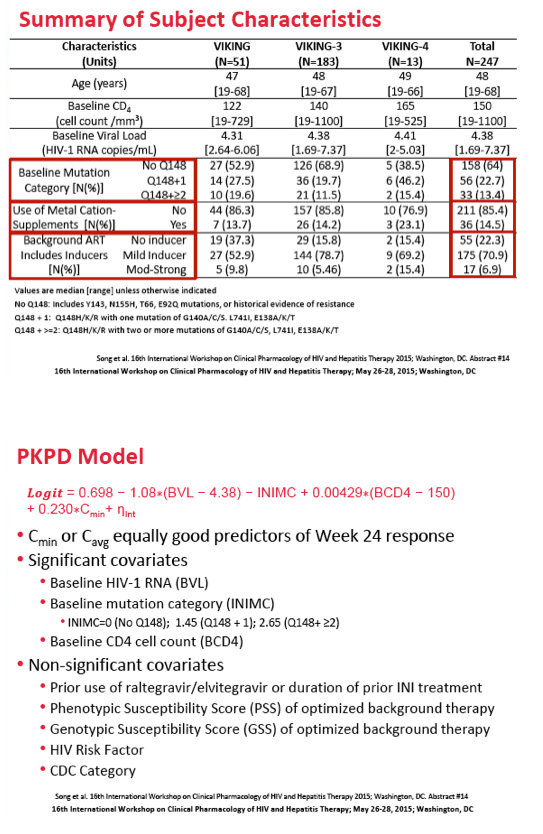
The analysis rested on sub-50-copy virologic response data at 24 weeks pooled from 247 trial participants. GSK investigators modeled response as a logistic function of dolutegravir exposure (minimum concentration or average concentration) and an array of covariates including viral load, CD4 count, baseline integrase resistance, phenotypic or genotypic susceptibility score of background antiretrovirals, prior use of raltegravir or elvitegravir, and duration of use. The GSK team validated the final model by comparing model predictions with responses observed in clinical trials. They used simulations based on observed and simulated patients to predict response rates to 100 mg of dolutegravir twice daily. The researchers ran two separate 100-mg simulations, one assuming a dose-proportional increase on dolutegravir concentrations, the other assuming a less than dose-proportional increase.
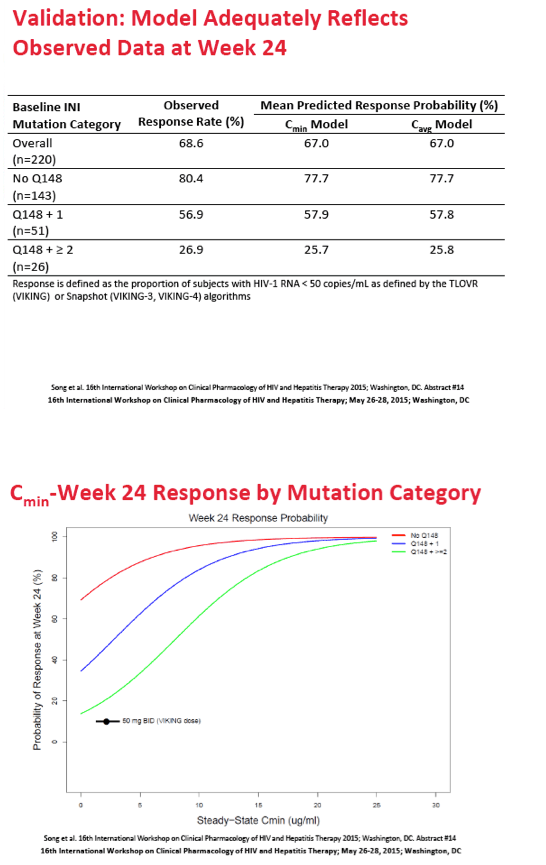
Overall observed sub-50-copy response at 24 weeks was 68.6%, while predicted responses were 67.0% with both the minimum concentration model and average concentration model. Modeled response rates also closely matched observed rates for participants with no Q148 mutation, with a Q148 mutation plus 1 more mutation, or with a Q148 mutation plus 2 or more other mutations.
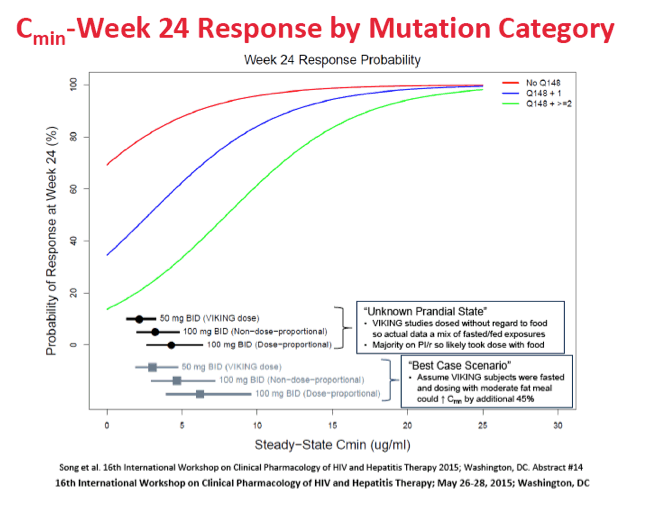
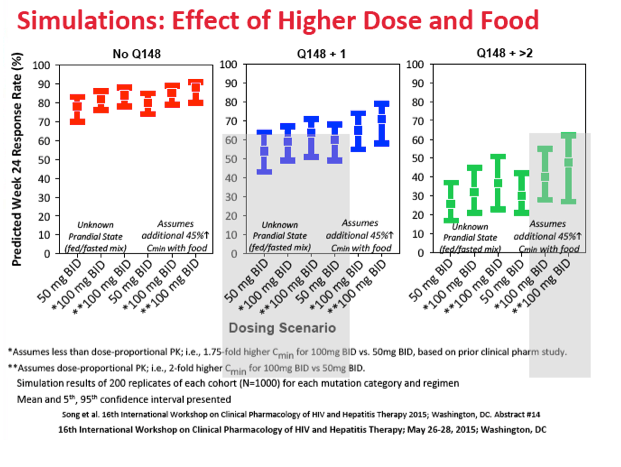
Simulations assuming dose-proportional PKs and a 45% increased exposure with food predicted that pushing the dolutegravir dose from 50 to 100 mg twice daily would boost the median 24-week 50-copy response rate from 80% to 88% in people with no mutations at integrase position Q148, from 60% to 71% in people with a Q148 mutation plus 1 other mutation, and from 30% to 48% in people with a Q148 mutation plus 2 or more other mutations..
But the GSK researchers found significant overlap in 90% prediction intervals between the 50- and 100-mg doses for all three mutation scenarios. Also, previous work showed less than dose-proportional dolutegravir pharmacokinetics when the dose jumps from 50 to 100 mg.
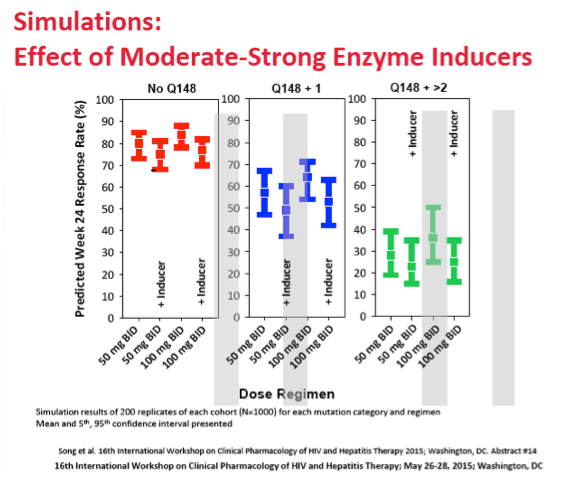
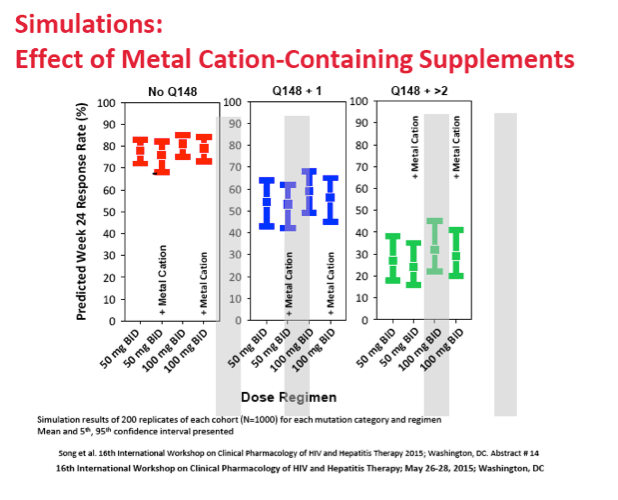
Taking dolutegravir with a strong UGT1A or CYP3A inducer lowered predicted response rates with the 50-mg twice-daily dose by 5% for no Q148 mutation, by 8% for Q148 plus 1 mutation, and by 5% for Q148 plus 2 or more mutations. Taking dolutegravir with a metal-cation dietary supplement had little impact on virologic response.
Considering all these results, the investigators believe this simulation supports the current recommendation of 50 mg twice daily for most patients with integrase inhibitor-resistant HIV. The simulations also confirmed the currently licensed twice-daily 50-mg dose in people taking moderate to strong UGT1A or CYP3A inducers or metal cation-containing vitamins.
Reference
1. Song I, Adkison K, Lovern M, et al. Pharmacokinetic-pharmacodynamic modeling and simulation of the virologic response of dolutegravir in HIV-infected patients with integrase inhibitor resistant virus. 16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy. May 26-28, 2015. Washington, DC. Abstract 14.
------------------
Pharmacokinetic-Pharmacodynamic Modeling and Simulation of the Virologic Response of Dolutegravir in HIV-Infected Patients with Integrase Inhibitor Resistant Virus
Reported by Jules Levin
16th International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy.
Washington DC, USA, 26-28 May 2015
I. Song1, K. Adkison1, M. Lovern2, J. Chiu2, J. Huang3, C. Vavro4, M. Ait-Khaled5, B. Wynne6, S. Min7
1GSK, CPMS, Research Triangle Park, USA; 2Quantitative Soluations, East Coast Office, Bridgewater, USA; 3GSK, Clinical Statistics, Toronto, Canada; 4GSK, ID Clinical Virology, Research Triangle Park, USA; 5GSK, MD ID, Stockley Park, United Kingdom; 6GSK, MD ID, Upper Marion, USA; 7GSK, MD ID, Research Triangle Park, USA
Background: Dolutegravir (DTG), a integrate inhibitor (INI), is currently approved at the 50mg twice daily (BID) dose for the treatment of HIV-1 infections in patients with integrase-resistant (INIr) virus based on demonstrated efficacy and safety in 3 studies (VIKING, VIKING-3, VIKING- 4). The objective of this analysis was to (1) describe the DTG exposure-Week 24 viral response relationship and (2) use model-based simulations to assess the impact of a higher dose of DTG or co-administration with food (known to increase DTG exposures) or enzyme inducing drugs and metal cation-containing dietary supplements (known to lower DTG plasma exposures) on response in the overall INI-r population, as well as subpopulations harboring the less sensitive Q148 viral mutations.
Materials & Methods: Using pooled data from 247 subjects, the probability of virology responder (response rate) at Week 24 as defined by Snapshot/TLOVR (HIV-1-RNA<50c/mL) was modeled as a logistic function of DTG plasma exposure (Cmin or Cavg estimated by an existing PopPK model) and covariates of interest including baseline viral load, baseline integrase resistance (fold change in IC50, integrase mutation category), phenotypic or genotypic susceptibility score of background antiretroviral therapy, prior raltegravir/elvitegravir use and duration of use, HIV risk factors, CDC category, baseline CD4+. Model building was performed in NONMEM and covariates were selected based on forward inclusion and backward elimination. The final model was validated by comparing the model predictions to the observed responses. Simulations based on observed and simulated subjects were performed to predict response rates for various scenarios including 100mg BID and dosing with food, metal cation-containing dietary supplements, or enzyme-inducing drugs.
Results: The logistic regression analysis based on either Cmin or Cmax arrived at the same final model, where baseline integrase mutation category, baseline viral load, and baseline CD4 were significant covariate effects. Based on the simulations, increasing dose from 50mg BID to 100mg BID assuming dose-proportional PK and a 45% increased exposure when dosed with food (best case scenario that conservatively assumed observed cases were fasted) is predicted to increase the Week 24 median predicted response rate from 80% to 88% for the 'No Q148' category, from 60% to 71% for the 'Q148+1' category, and from 30% to 48% for the 'Q148+32' category, respectively, using the Cmin-based model.
However there was significant overlap in the 90% prediction intervals between 50mg BID and 100mg BID for all three mutation categories, and DTG PK was previously shown to be less than dose-proportional from 50mg to 100mg. Coadministration with strong inducers resulted in lower predicted response rates for the 50mg BID regimen by 5%, 8%, and 5% in the No Q148, Q148 + 1, and Q148 + ≥2 mutation categories. Co-administration with metal-cation dietary supplements was predicted to have a negligible impact on virologic response.
Conclusions: A logistic regression model was developed and validated to describe the relationship between the virologic response at Week 24 and DTG exposure in INI-r subjects. The simulation results support the current dose recommendation of 50mg BID for the treatment of HIV infection in patients with integrase-resistant viruses.

|
| |
|
 |
 |
|
|