 |
 |
 |
| |
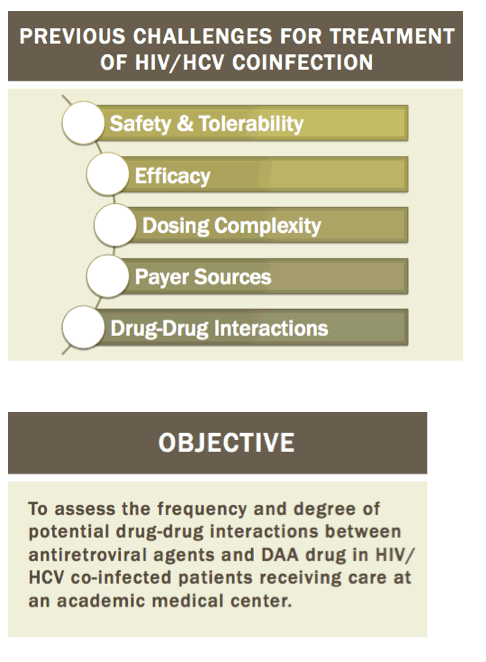
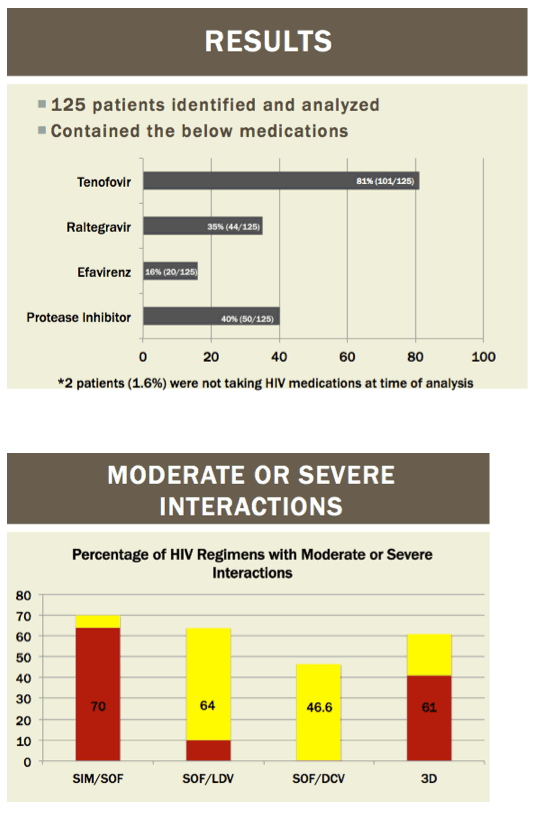
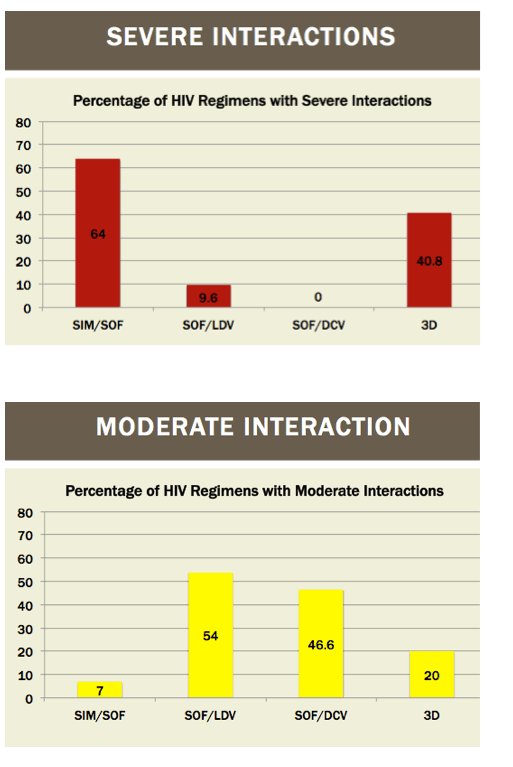
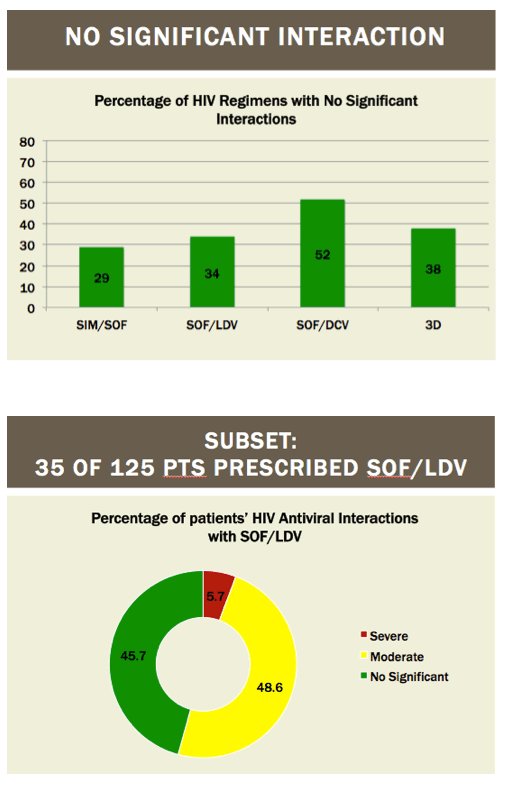
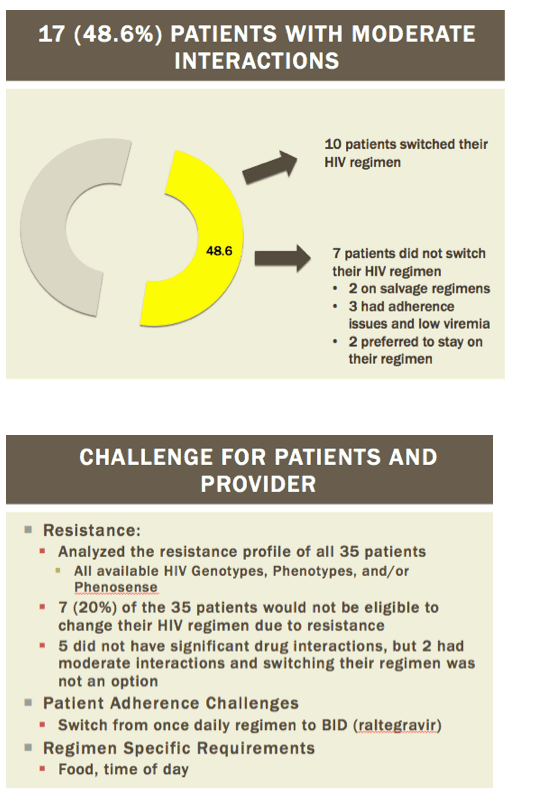
Readying HIV/HCV Coinfected Patients for HCV Treatment: Occurrence and Management of Antiviral Interactions....[can patients switch their HIV regimen?]
|
| |
| |
Reported by Jules Levin
16th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy, May 26-28, 2015, Washington, DC
Jacob Langness1, PharmD, BCPS, Bayli Larson2, PharmD Candidate, Joshua Bayer1, PharmD, BCPS, Maya Rogers1, MD, Jennifer Kiser2, PharmD
1University of Colorado Hospital, Aurora, CO USA
2Skaggs School of Pharmacy and Pharmaceutical Sciences, Aurora, CO USA

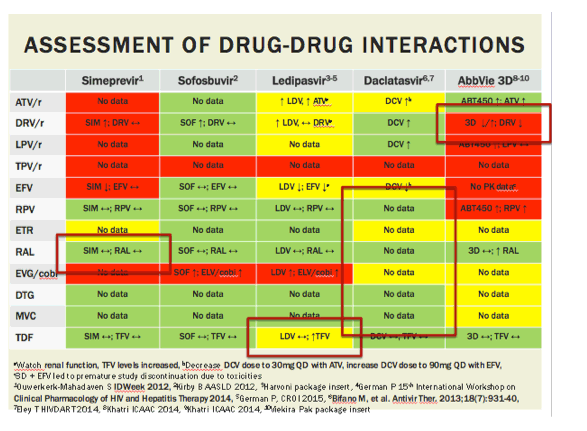
aWatch renal function, TFV levels increased, bDecrease DCV dose to 30mg QD with ATV, increase DCV dose to 90mg QD with EFV,
c3D + EFV led to premature study discontinuation due to toxicities
1Ouwerkerk-Mahadaven S IDWeek 2012, 2Kirby B AASLD 2012, 3Harvoni package insert, 4German P 15th International Workshop on Clinical Pharmacology of HIV and Hepatitis Therapy 2014, 5German P, CROI 2015, 6Bifano M, et al. Antivir Ther. 2013;18(7):931-40, 7Eley T HIVDART 2014, 8Khatri ICAAC 2014, 9Khatri ICAAC 2014, 10Viekira Pak package insert
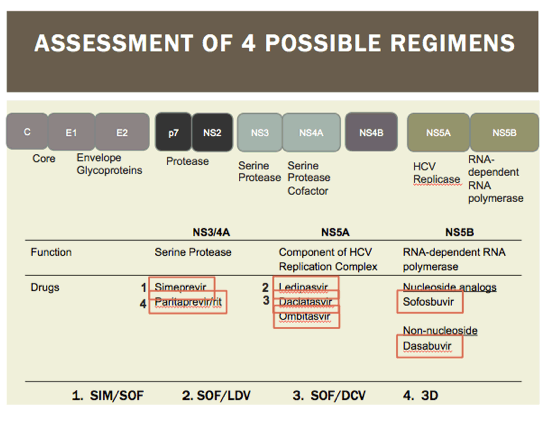
The E2 region of HCV virus is similar the the V3 loop (gp120) of HIV virus. This is hypervariable in HCV patients. In coinfected patients, there is a known increase in variability
|
| |
|
 |
 |
|
|