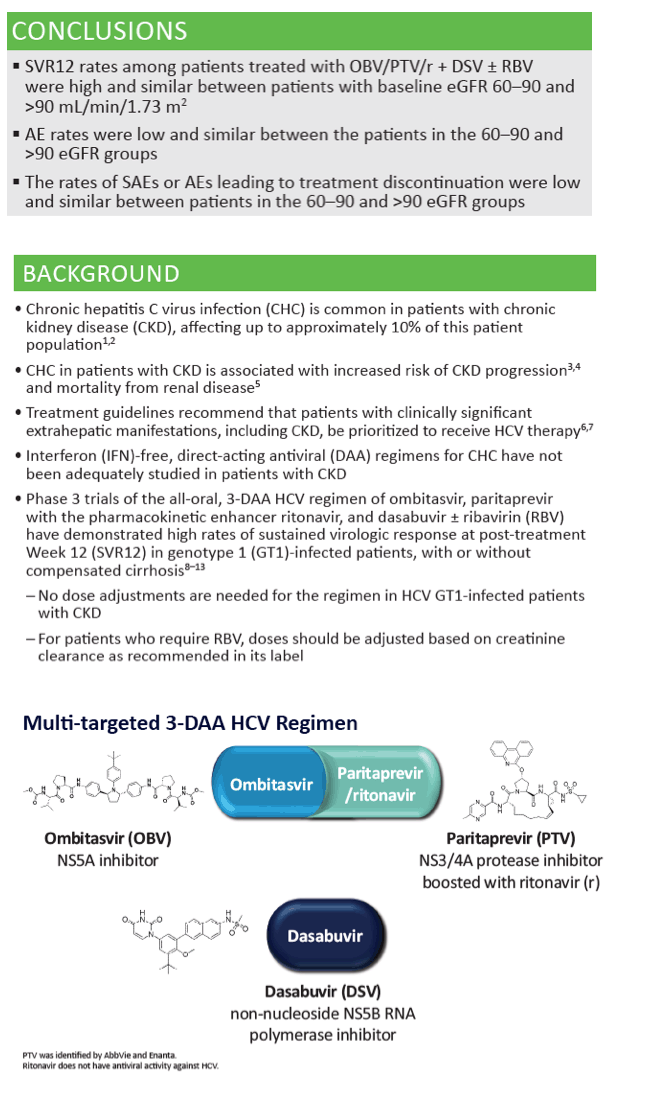
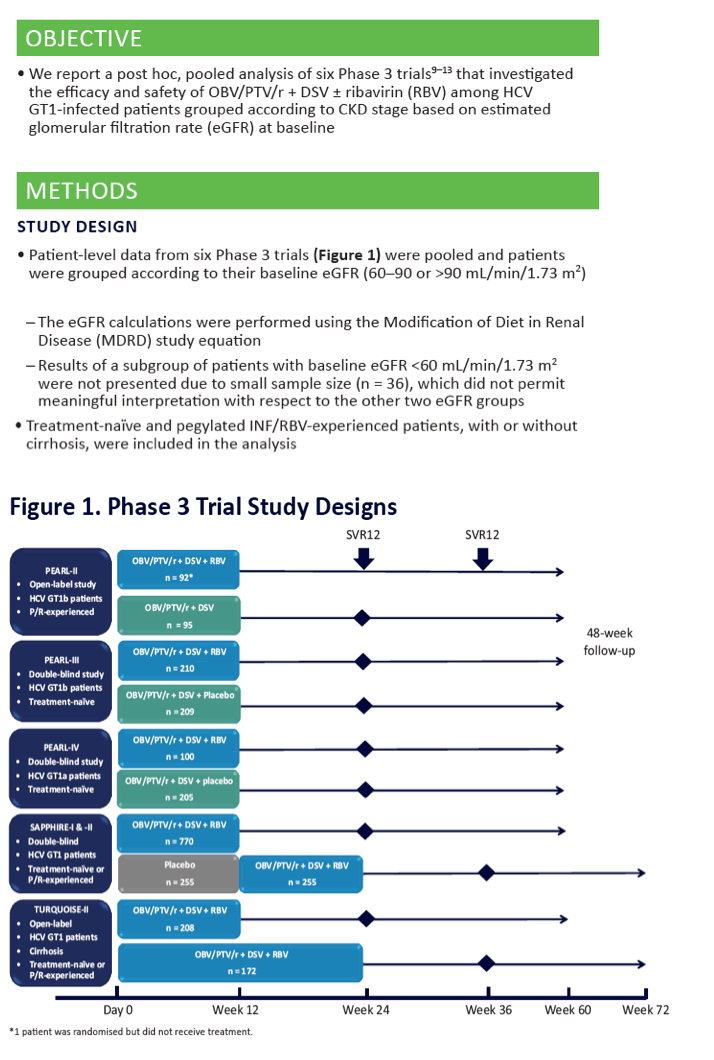
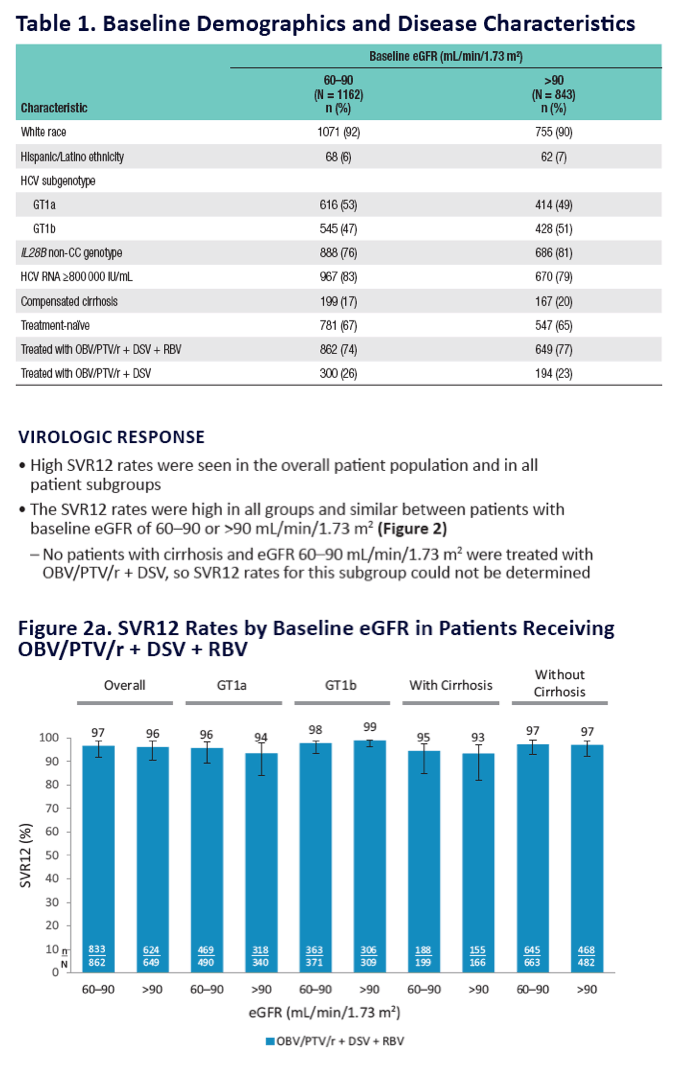
 |
 |
 |
| |
(HepDart) Efficacy and Safety of Ombitasvir/Paritaprevir/r + Dasabuvir ± Ribavirin According to Baseline Renal Function: Analysis of 2005 Patients Enrolled in Six Phase 3 Trials
|
| |
| |
Reported by jules Levin
HEP DART 2015, December 6-10, 2015, Wailea, Hawaii
Mark S Sulkowski1, Paul J Pockros2, Ira M Jacobson3, David E Bernstein4, Fernando Tatsch5, Boris Renjifo5, Deli Wang5, Daniel E Cohen5, Paul Martin6
1Viral Hepatitis Center, Johns Hopkins University, Baltimore, Maryland, United States; 2Division of Gastroenterology/Hepatology, Scripps Clinic, La Jolla, California, United States; 3Icahn School of Medicine at Mount Sinai, New York, New York, United States; 4Division of Hepatology, North Shore - LIJ Hofstra School of Medicine, Manhasset, New York, United States; 5AbbVie Inc., North Chicago, Illinois, United States; 6Division of Hepatology, School of Medicine, University of Miami, Miami, Florida, United States
These studies presented at AASLD were presented at HepDart too:
AASLD: SURVEYOR-II: High SVR4 Rates Achieved With the Next Generation NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 in Non-Cirrhotic Treatment-Naïve and Treatment-Experienced Patients With HCV Genotype 2 Infection - (12/01/15)
AASLD: SURVEYOR-II: High SVR4 Rates Achieved With the Next Generation NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 in Non-Cirrhotic Treatment-Naïve and Treatment-Experienced Patients With HCV Genotype 3 Infection - (12/01/15)
AASLD: 100% SVR4 in HCV Genotype 1 Non-Cirrhotic Treatment-Naïve or -Experienced Patients With the Combination of ABT-493 and ABT-530 for 8 Weeks (SURVEYOR-I)....[8 weeks] - (11/17/15)
AASLD: SURVEYOR-I: 98% - 100% SVR4 in HCV Genotype 1 Non-Cirrhotic Treatment-Naïve or Pegylated Interferon/Ribavirin Null-Responders with the Combination of the Next Generation NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 - (11/16/15)
AASLD: ABBVIE ANNOUNCES HIGH SUSTAINED VIROLOGIC RESPONSE RATES IN PHASE 2 STUDIES WITH PAN-GENOTYPIC, INVESTIGATIONAL REGIMEN IN PATIENTS WITH CHRONIC HEPATITIS C - (11/19/15)
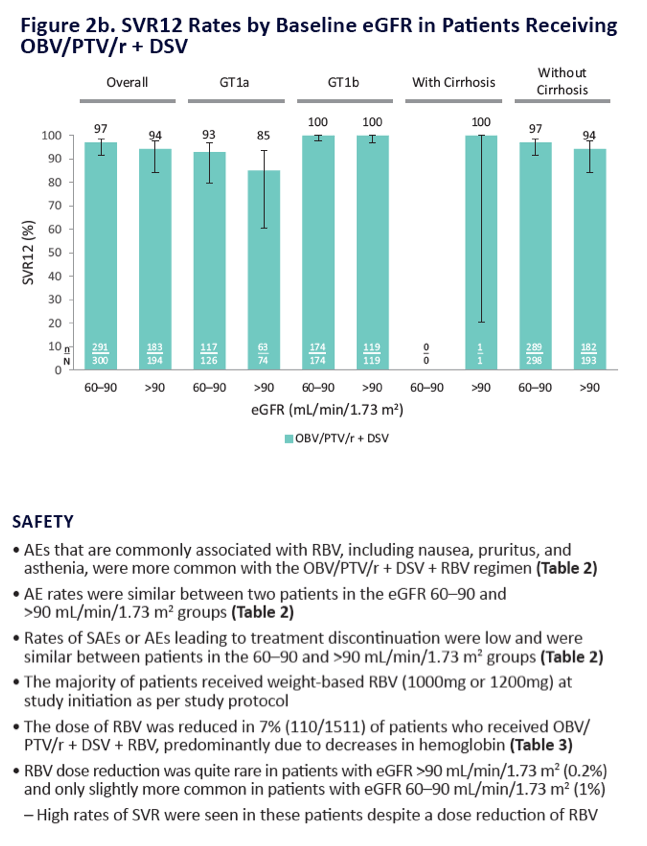
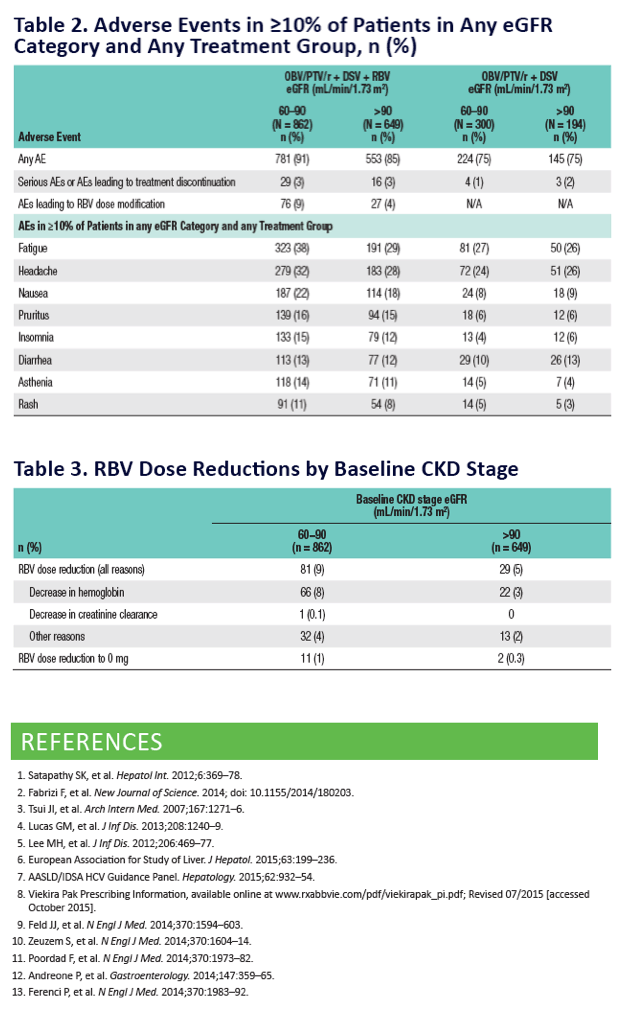
|
| |
|
 |
 |
|
|