 |
 |
 |
| |
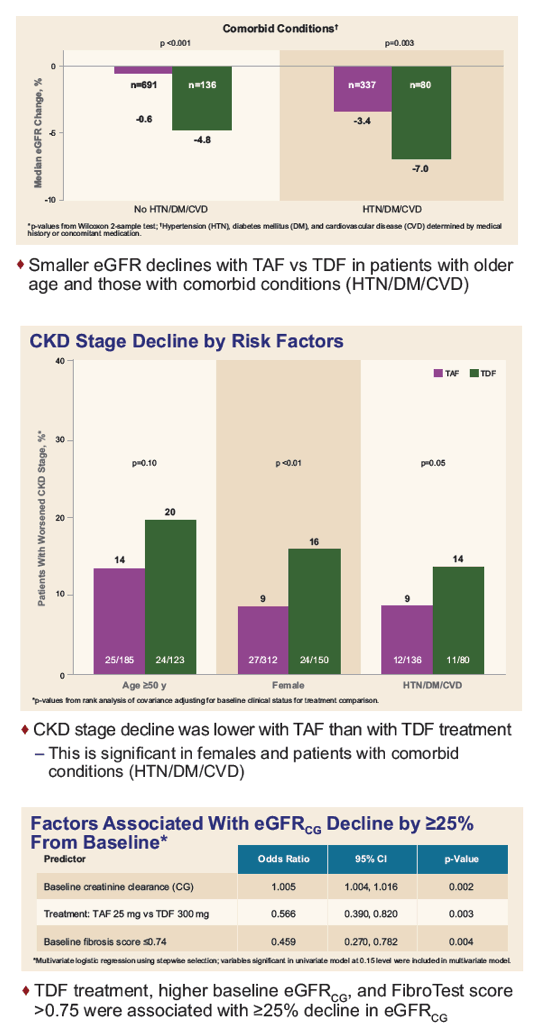
Improved Renal Laboratory Parameters in CHB Patients
Treated With TAF Compared With TDF
|
| |
| |
Reported by Jules Levin
AASLD 2016 Nov 11-15 Boston, MA
Kosh Agarwal, 1 Norihiro Furusyo, 2 Kwan-Soo Byun, 3 Jae-Seok Hwang, 4 John F. Flaherty, 5 Kyungpil Kim, 5 Anuj Gaggar, 5 G. Mani Subramanian, 5 Maciej S. Jablkowski, 6 Alexey A. Yakovlev, 7 Huy N. Trinh, 8 Maria Buti 9
1Institute of Liver Studies, Kings College Hospital, London, UK; 2KyUshU University Hospital, Fukuoka, Japan; 3Korea University, Seoul, Korea; 4Keimyung University Dongsan Medical Center, Daegu, Korea; 5Gilead Sciences, Inc., Foster City, CA;
6Oddzia Obserwacyjno-Zakazny, Lodz, Poland; 7S.P. Botkin Clinical Infectious Diseases Hospital, St. Petersburg, Russia; 8Silicon Valley Research Institute, San Jose, CA; 9Vall d'Hebron Hospital, Barcelona, Spain
ASM/ICAAC: Tenofovir Alafenamide in Participants with
Diabetes and Renal Impairment:
Renal Safety Through 96 Weeks......http://www.natap.org/2016/HIV/062116_03.htm

1. Lampertico P, et al. Aliment Pharmacol Ther 2016;44:16-34; 2. Marcellin P, et al. AASLD 2014, oral; 3. Hall AM, et al. Am J Kidney Dis 2011;57:773-80;
4. Babusis D, et al. Mol Pharm 2013;10:459-66; 5. Lee WA, et al. Antimicrob Agents Chemother 2005;49:1898-906; 6. Murakami E, et al. Antimicrob
Agents Chemother 2015;59:3563-9; 7. Agarwal K, et al. J Hepatol 2015;62:533-40; 8. Sax P, et al. Lancet 2015;385:2606-15; 9. Buti M, et al. Lancet
Gastroenterol Hepatol In press; 10. Chan HLY, et al. Lancet Gastroenterol Hepatol In press; 11. Mills A, et al. Lancet Infect Dis 2016;16:43-52; 12. Terrault NA,
et al. Hepatology 2016;63:261-83; 13. National Kidney Foundation. Am J Kidney Dis 2012;60:850-86; 14. KDIGO 2012 clinical practice guideline for
the evaluation and management of chronic kidney disease. Kidney Int 2013;3 (suppl):1-150.
|
| |
|
 |
 |
|
|