 |
 |
 |
| |
Pitavastatin: an overview of the LIVES study
|
| |
| |
John JP Kastelein* & Marjet Braamskamp
The Livalo® (Livazo® in Europe) Effectiveness and Safety (LIVES) study, a postmarketing surveillance trial conducted in 20,279 Japanese patients with hypercholesterolemia, is the largest trial evaluating the efficacy and safety of pitavastatin 1-4 mg/day. Elderly patients (aged ≥65 years) accounted for approximately 50% of participants, and about 75% of all patients had concomitant diseases, including hypertension and Type 2 diabetes. During 2 years of follow-up, only 10.4% of patients developed adverse drug reactions (ADRs), most of which were mild in severity. No previously unknown ADRs were identified, and laboratory abnormalities such as elevated serum creatine phosphokinase (2.74%), alanine aminotransferase (1.79%), aspartate aminotransferase (1.5%) and myalgia (1.08%) represented the most common ADRs. Importantly, the ADR incidence did not differ significantly based on either concomitant drug use or age. Furthermore, subgroup analyses of the LIVES study showed that pitavastatin has beneficial effects on renal function (increased estimated glomerular filtration rate) and glycemic control (decreased HbA1c) in hypercholesterolemic patients with chronic kidney disease and diabetes mellitus, respectively. With regard to efficacy, there was a significant reduction in LDL-C levels by 4 weeks after the start of treatment, which remained stable throughout the study period. Pitavastatin significantly and continuously increased HDL-C over 2 years. Interestingly, the percentage increase in HDL-C was higher in patients with baseline HDL-C <40 mg/dl. Similarly, a 24.2% reduction in triglycerides was observed in patients with baseline values >150 mg/dl after 2 years of pitavastatin treatment. These outcomes have been confirmed in the LIVES extension study, a 3-year follow-up of approximately 7000 patients who were enrolled in LIVES and treated with pitavastatin for >2 years. The LIVES extension study also found that the risk of cardiovascular, cerebrovascular and sudden cardiac death events was significantly reduced in patients who achieved on-treatment HDL-C and LDL-C target levels compared with patients not achieving lipid goals. In conclusion, pitavastatin has an excellent safety profile, even in polymedicated and elderly patients and, in addition to lowering LDL-C and triglyceride levels it increases HDL-C, which may be predictive of residual cardiovascular risk among patients on lipid-lowering medications, thus providing effective and sustained improvements in the atherogenic profile.
Figure 1. Change from baseline in lipid profile after 3 months of treatment with pitavastatin 1-4 mg daily in the LIVES study.
All p < 0.001 versus baseline.
BL: Baseline.
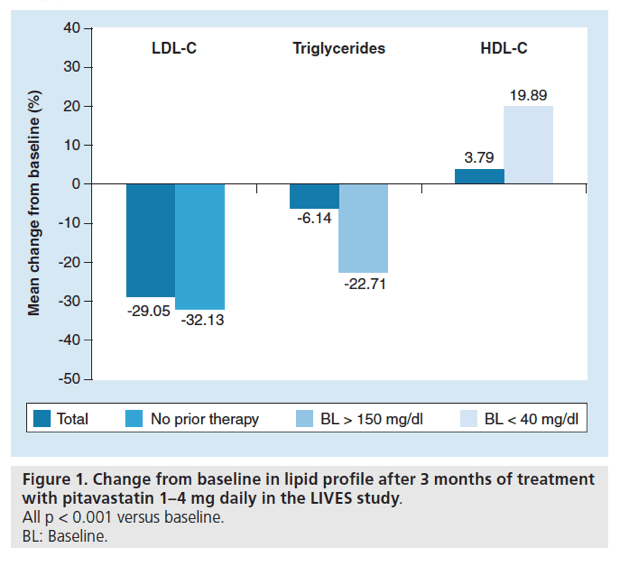
Figure 2. Change from baseline in lipid values after 104 weeks of pitavastatin treatment in patients with hypercholesterolemia.
All changes p < 0.0001 vs baseline.
BL: Baseline; TG: Triglyceride.
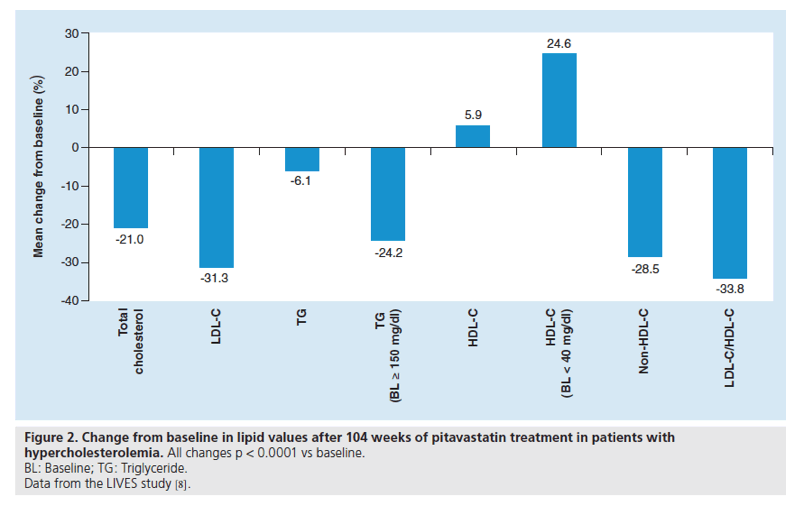
Figure 3. Effect of pitavastatin on HDL-C levels after switching from other statins.
*p < 0.01 (two sample t-test); **p < 0.01 (vs at baseline).
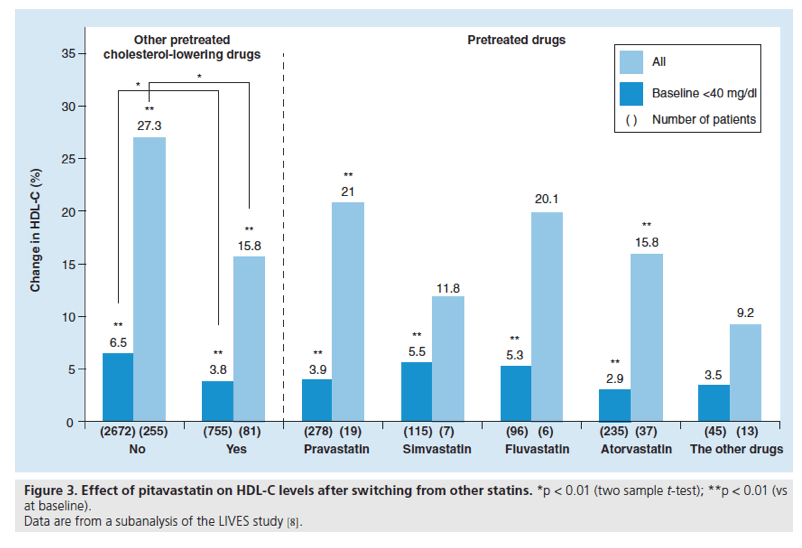
Figure 4. Effect of pitavastatin on glycemic control.
Data are taken from a subanalysis of the LIVES study (n = 1197).
SD: Standard deviation.
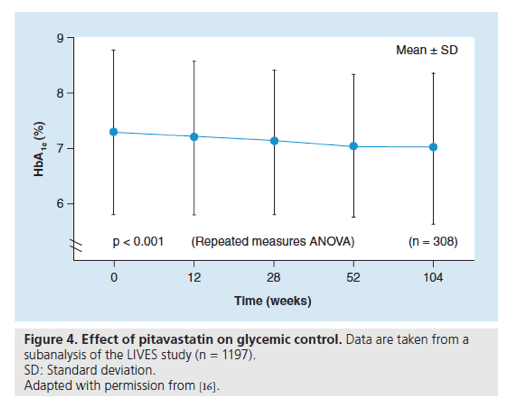
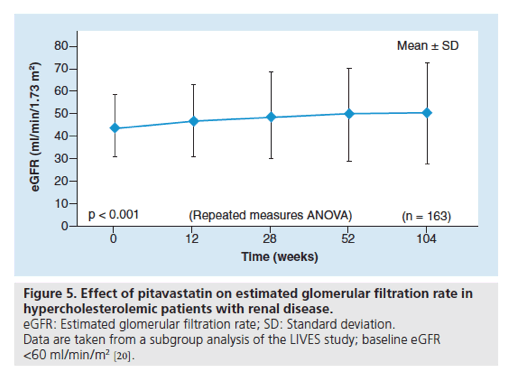
Figure 5. Effect of pitavastatin on estimated glomerular filtration rate in hypercholesterolemic patients with renal disease.
eGFR: Estimated glomerular filtration rate; SD: Standard deviation.
Data are taken from a subgroup analysis of the LIVES study; baseline eGFR <60 ml/min/m2
Numerous new approaches are being pursued in the area of research and development of lipid-modifying drugs, including: cholesterol absorption inhibitors, squalene synthase inhibitors, ApoB mRNA antisense drugs, microsomal triglyceride (TG) transfer protein inhibitors, thyroxin receptor agonists and PCSK9 inhibitors [1-4]. In addition to these new and exciting approaches, the newest HMG-CoA reductase inhibitor (statin), pitavastatin, is also the subject of continuing intensive clinical and scientific research, despite being available in Japan since September 2003 and continuing to be marketed throughout the world.
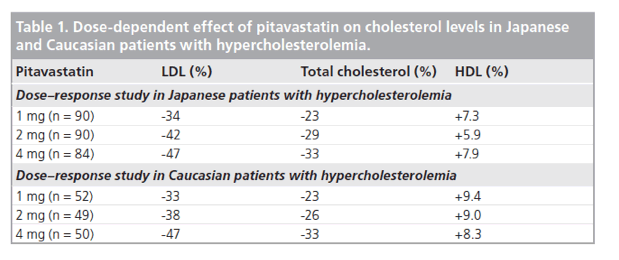
Although the majority of clinical research and clinical studies with pitavastatin have been conducted in Japan, it is interesting to note that separate dose-response studies in Japanese and Caucasian patients demonstrate that pitavastatin possesses very similar effects on cholesterol levels in the two different populations (Table 1). Within the approved dosage range, pitavastatin 1-4 mg lowers LDL-C and total cholesterol, and increases HDL-C to a numerically similar degree in Japanese and Caucasian patients.
Furthermore, Warrington and colleagues have recently published data showing that the EU and Japanese formulations of pitavastatin are pharmacokinetically bioequivalent and, when bodyweight differences are taken into account, there are no clinically relevant differences in exposure to pitavastatin between Caucasian and Japanese patients [5]. Data were obtained from an open-label, single-dose, two-way crossover study in healthy men (Caucasian: n = 48; Japanese: n = 12). Pitavastatin maximum plasma concentrations of 22.9 and 22.5 ng/ml, and area under the concentration-time curve values of 44.4 ngμh/ml and 45.9 ngμh/ml were obtained in Caucasian and Japanese men, respectively.
The similarity in pharmacokinetic parameters and effects on cholesterol levels in Caucasian and Japanese populations receiving pitavastatin are not observed consistently with other statins. Furthermore, the implications of these observations are broad-ranging as they allow direct correlation of clinical outcomes derived from large Japanese studies to Caucasian populations.
Livalo® Effectiveness and Safety (LIVES) Study
The Livalo® (Livazo® in Europe) Effectiveness and Safety (LIVES) study is a large, long-term (a fixed 3-month period with a 2-year follow-up), prospective, observational, postmarketing surveillance study of pitavastatin that has been conducted in 'real life' clinical practice in Japan [6]. Patients were enrolled into the study from December 2003 to March 2005. The primary objectives of the LIVES study were to evaluate the incidence and pattern of known and any previously unknown adverse drug reactions (ADRs), and to identify clinical factors that might have an impact on the overall safety and efficacy of pitavastatin in patients with hypercholesterolemia or familial hypercholesterolemia. An extension of the LIVES study, the LIVES study extension, is evaluating 5-year follow-up of individuals from the original LIVES study (see later section 'LIVES study extension').
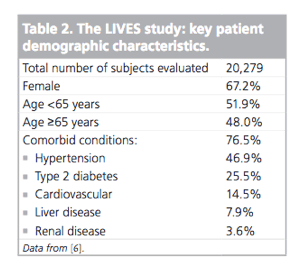
Of 20,279 patients recruited into the LIVES study, 19,925 were analyzed for drug safety and 18,031 patients were available for effectiveness analyses (i.e., approximately 40,000 patient exposure years across the 2-year follow-up). The study included patients with Type 2 diabetes (n = 5133) and impaired renal function (n = 958). The most frequent daily dosages of pitavastatin were: 1 mg (40%), 2 mg (58.4%) or 4 mg (0.6%). Key baseline patient demographic characteristics are presented in Table 2 [6].
After 3 months of treatment with pitavastatin (as mandated by Japanese regulations), statistically significant (all p < 0.001) changes in LDL-C, HDL-C and TG levels were observed in all patients and in subgroups that included patients with/without prior drug therapy (LDL-C), patients with baseline HDL-C levels <40 mg/dl (HDL-C) and patients with TG levels >150 mg/dl (TG) (Figure 1). Indeed, peak serum LDL-C reduction occurred within 4 weeks of pitavastatin treatment initiation [6].
At 2 years, 2069 of 19,925 (10.4%) patients evaluable for safety parameters experienced ADRs; the vast majority of ADRs were mild (1735 patients) or moderate (307), with serious ADRs being reported in only 27 patients [6]. It is well documented that the most potentially concerning ADRs reported with statin therapy are myalgia, rhabdomyolysis and myopathy, associated with increases in creatine phosphokinase, alanine aminotransferase (ALT) and aspartate aminotransferase (AST); nevertheless, the clinical relevance (if any) of statin-associated increases in ALT and AST is currently unknown. In the LIVES study, myalgia (1.08%) and myopathy (0.03%) occurred with a very low incidence, and similar low incidences of elevated creatine phosphokinase (2.74%), ALT (1.79%) and AST (1.50%) were also reported [6].
In order to evaluate the rhabdomyolysis/myopathy/muscle symptoms in more depth, two logistic regression analyses of data from the LIVES study (Japan) and pooled Phase II/III data from European and US clinical trials have been performed. Data from the LIVES study for rhabdomyolysis/myopathy/muscle symptoms, show that increasing the pitavastatin dose was not associated with any statistically significant increase in symptoms, but there was a greater risk of symptoms in males versus females (p = 0.0025), with some concomitant drug use and increasing renal impairment [Kowa, Pers. Comm.]. Similar data were reported from the pooled data from European and US clinical trials [7]: there was no association with pitavastatin dose in the therapeutic range; women were at lower risk than men; renal impairment and some concomitant drug use was associated with an increased risk; there was no association with patient age.
LIVES study: sub analyses
Beyond the LIVES study data reported from the initial database [6], several subgroup analyses have been performed that provide greater detail with respect to specific aspects of the role of pitavastatin in patients with hypercholesterolemia.
• Effect on HDL-C levels
Using data from the LIVES study database, Teramoto et al. evaluated the long-term (104 weeks) effects of pitavastatin on HDL-C levels (as well as on LDL-C and TGs) [8]. Over the course of 104 weeks the mean increase from baseline in HDL-C levels was 5.9% (p < 0.0001) in all patients; in patients with baseline HDL-C <40 mg/dl, HDL-C levels increased by 24.6% (p < 0.0001) after 104 weeks of treatment with pitavastatin. In a time-course analysis, HDL-C levels in patients with low baseline HDL-C were continuously elevated by pitavastatin (increased by 14% and 25% at 12 and 104 weeks, respectively) [8]. Compared with baseline values, there were also significant reductions (all p < 0.0001) at week 104 in total cholesterol, LDL-C, TGs (total and in patients with a baseline value ≥150 mg/dl), non-HDL-C and LDL-C/HDL-C ratio (Figure 2).
Extending these observed HDL-C increases with pitavastatin over a 5-year follow-up period (the LIVES extension study), those patients with baseline HDL-C <40 mg/dl (mean baseline HDL-C level: 35.2 mg/dl) showed continuously increasing mean HDL-C levels over 5 years that increased by 29% from baseline (mean 5-year HDL-C level: 44.9 mg/dl) [9]. It can be postulated that this continuous HDL-C elevating effect of pitavastatin is due to increased expression of apolipoprotein-A1 in the liver and intestine.
Furthermore, pitavastatin produced a significant increase in HDL-C in patients who were switched from other statins (Figure 3): pravastatin (a 21.0% increase in HDL-C), simvastatin (11.8%), fluvastatin (20.1%) and atorvastatin (15.8%). Multivariate analysis showed BMI, diabetes, liver disease and pretreatment with other cholesterol-lowering drugs to be significant factors influencing changes in HDL-C [8].
• Effect on glycemic control
Recently, a large body of evidence has emerged that indicates a slightly increased incidence of Type 2 diabetes with statin therapy [10-15]. Whilst the underlying mechanisms relating to an increased risk of diabetes remain unexplained, it is important to evaluate the effects of new statins on glycemic control. The effect of pitavastatin on glycemic control has been evaluated in a subgroup analysis of patients from the LIVES study; HbA1c data were available for 1197 hypercholesterolemic patients with diabetes at baseline and at 104 weeks. In patients not receiving antidiabetic medication at baseline, the mean change in HbA1c levels was +0.077% (6.15 to 6.22%; p = 0.13 [n = 205]); in patients receiving antidiabetes therapy at baseline, the mean change in HbA1c levels was -0.28% (7.51 to 7.23%; p < 0.001 [n = 922]). In the time-course analysis [16], HbA1c levels decreased gradually by 0.28% over the 104 week observation period (p < 0.001, repeated measures ANOVA) (Figure 4). A multivariate analysis identified the presence/absence of antidiabetes therapy and percentage changes in TGs and LDL-C as clinical factors influencing the decrease in HbA1c levels induced by pitavastatin, and it would appear to be clinically reasonable to assume that there may be a relationship between reduced TG levels and decreased HbA1c levels. Whilst recognizing that the trial was not active- or placebo-controlled, the LIVES study subgroup analysis, nevertheless, suggests that pitavastatin appears to have no significant adverse effect on glucose metabolism in normoglycemic or diabetic individuals.
• Effect in patients with chronic kidney disease
Chronic kidney disease (CKD) is known to be associated with an increased risk of cardiovascular disease [17-19]. Hypercholesterolemic patients with CKD represent an important population of patients who are indicated for statin medication. The effect of pitavastatin on the estimated glomerular filtration rate (eGFR) has been evaluated in hypercholesterolemic patients with CKD in the LIVES study [20]. Of the 19,925 patients evaluable for safety parameters in the LIVES study, 3119 patients were analyzed to evaluate the effects of pitavastatin for 104 weeks on eGFR; of these patients, 958 (30.7%) had a baseline eGFR <60 ml/min/1.73m2. Compared with baseline, a significant increase in eGFR (mean: +5.4 ml/min/1.73m2; p < 0.001) was observed after 104 weeks of treatment with pitavastatin. An analysis of time-course changes in eGFR after pitavastatin treatment showed a consistently elevated response from baseline: eGFR at 12 weeks (+2.4 ml/min/1.73m2 [4.8% increase]) and at 104 weeks (+5.6 ml/min/1.73m2 [10.5% increase]; p < 0.001; Figure 5) [20].
LIVES study extension
As discussed briefly previously, a 3-year extension to the original 2-year LIVES study has been performed to allow an overall 5-year surveillance of cardiovascular events, cerebrovascular events and sudden death in patients receiving daily pitavastatin 1, 2 or 4 mg for >2 years. Approximately 7000 patients were enrolled up to March 2010 and followed throughout the LIVES study extension (an open-label, observational, postmarketing surveillance study with no control group). Data from the LIVES extension study should allow an insight into outcomes evidence with pitavastatin - published data from this 5-year analysis are awaited with anticipation. Importantly, a preliminary analysis of the relationship between on-treatment LDL-C and HDL-C levels and cardiovascular, cerebrovascular and sudden cardiac death (SCD) events, suggests that patients who achieve lipid goals with pitavastatin have significantly greater survival than patients not achieving lipid goals [9]. Based on Japanese LDL-C and HDL-C treatment goals [21], 71.1% of patients at both LDL-C and HDL-C goal had a significantly (p < 0.0001) lower incidence of cardiovascular, cerebrovascular and SCD events than patients (4.8%) with LDL-C at goal/HDL-C not at goal, patients (21.5%) with LDL-C not at goal/HDL-C at goal, and patients (2.6%) with both LDL-C and HDL-C not at goal. Whilst these data are obtained from an open-label, observational, postmarketing surveillance study with no control group, and therefore need to be viewed with a degree of caution, they provide an important hypothesis-generating observation suggesting that lowering LDL-C and elevating HDL-C to target levels with pitavastatin treatment may be associated with fewer cardiovascular, cerebrovascular and SCD events.
Conclusions
Pharmacokinetic and pharmacodynamic studies suggest that the efficacy, safety and tolerability of pitavastatin are similar across the dose range in Japanese and Caucasian individuals. The large database of the LIVES study, extending to 5-years of follow-up, shows that in 'real life' clinical practice in Japan, pitavastatin has a very favorable safety profile in terms of liver and muscle adverse events, an absence of diabetogenic properties and improves renal function. After 2 years of treatment with pitavastatin, HDL-C levels in the overall population increased by 6% and, in patients with low (<40 mg/dl) baseline HDL-C levels, the elevation was 25%. Furthermore, data from the LIVES extension study suggest that patients treated with pitavastatin who achieve on-treatment goals for both HDL-C and LDL-C levels, exhibit fewer cardiovascular, cerebrovascular and SCD events than those not at goal.
|
| |
|
 |
 |
|
|