 |
 |
 |
| |
An assessment by the Statin Muscle Safety Task Force: 2014 update
|
| |
| |
Download the PDF here
2014 Statin Safety Task Force
Provider recommendations to patients
It is not uncommon to find media, Internet, and book-promoting sources that warn against the use of statins because of concerns of liver toxicity (among other reported reasons). It is true that statins may potentially cause very rare hepatic adverse experiences. However, these extremely rare occurrences should not detract from the potential benefit of proven reduction in CHD risk, which has far greater health implications than the possibility of serious liver injury. The objective evidence strongly suggests that for patients at risk for CHD, the risks of not taking the statin outweigh the risks of taking the statin. Patients should appreciate that based on data from objective, large-scale clinical trials, statins are not only remarkably safe, but, most of all, statins reduce the chances of CHD events, including myocardial infarction (heart attack), stroke, and revascularization procedures among patients with and without manifest vascular disease. Statins have been shown to reduce deaths from these diseases.21, 22
-------------------
Highlights
⋅Differential diagnosis of myalgia occurring during statin therapy.
⋅Nosology for statin-associated muscle adverse events that provides definitions with clinical utility.
⋅Myalgia clinical index score.
⋅Recommendations for neuromuscular testing.
Abstract
The National Lipid Association's Muscle Safety Expert Panel was charged with the duty of examining the definitions for statin-associated muscle adverse events, development of a clinical index to assess myalgia, and the use of diagnostic neuromuscular studies to investigate muscle adverse events. We provide guidance as to when a patient should be considered for referral to neuromuscular specialists and indications for the performance of a skeletal muscle biopsy. Based on this review of evidence, we developed an algorithm for the evaluation and treatment of patients who may be intolerant to statins as the result of adverse muscle events. The panel was composed of clinical cardiologists, clinical lipidologists, an exercise physiologist, and a neuromuscular specialist.
An assessment by the Statin Muscle Safety Task Force: 2014 update
Muscle complaints represent the most frequent adverse reports among patients treated with statins. These complaints occur in a population that often has musculoskeletal pain and dysfunction in the absence of statins; therefore, the careful assessment of such adverse reports is essential to provide the most efficacious cardiovascular risk management. The spectrum of statin-associated muscle toxicity, often termed "statin-associated myopathy," is considered to include several distinct entities that may overlap in clinical presentation (Table 1); however, there is no evidence that the constellation of muscle adverse reports is a continuum that begins with myalgia and progress to more severe manifestations of myopathy. Thus, each statin-associated muscle event must be categorized with the use of standard definitions to minimize misinterpretation of the etiology and misclassification of muscle adverse reports in those using statins for prevention of cardiovascular diseases. These associations are usually temporal, and causality is often very hard to prove. It should also be noted that statins are not the only chemical entity that can induce myopathic changes. Other common drugs include substances of abuse (alcohol, cocaine, opioids), neuroleptics and psychotropic agents (haloperidol, risperidone), immunosuppressants (cyclosporine A, azathioprine), antiviral agents (zidovudine, ritonavir, didanosine), analgesics and anti-inflammatory drugs (salicylates, nonsteroidal anti-inflammatory drugs, glucocorticoids), fibrates (gemfibrozil, fenofibrate), anesthetics and neuromuscular blocking agents (propofol, ketamine, succinylcholine). Furthermore, genetic, infectious, and immune disorders can present with muscle signs and symptoms. Misdiagnosis may preclude unnecessarily the future use of this class of efficacious agents in a given patient and delay appropriate treatment of other unrelated myopathic disorders.
--------------------------------
⋅3. Are statin-associated muscle complaints altered by acute and chronic physical activity?
ANSWER: Yes.
STRENGTH OF RECOMMENDATION: A (Strong).
QUALITY OF EVIDENCE: Moderate.
EXPLANATION: Statins are less tolerated in physically active individuals. For example, only 20% of 22 professional athletes with familial hypercholesterolemia were shown to ultimately tolerate statin therapy despite multiple trials with multiple medications.27 In the PRIMO study, incidence of muscle pain with statin therapy increased with the level of physical activity from 10.8% in those engaging in leisure-type physical activity to 14.7% in those regularly engaging in vigorous activity, suggesting that statin-associated muscle side effects are provoked by physical activity.10 Moreover, several studies indicate that acute physical activity increases the CK response to exercise in participants on statins. For example, CK levels obtained 24 and 48 hours after a treadmill exercise test were 62% and 77% greater, respectively, in men treated with 4 weeks of lovastatin 40 mg daily than in age-matched, placebo-treated subjects.28 After intense and prolonged exercise in the 2011 Boston Marathon, exercise-related increase in CK levels at 24 hours were greater in statin users than controls after adjustment for changes in plasma volume.29 Several other studies, however, have failed to find an effect of statin therapy on exercise-associated muscle damage,30 possibly because of differences in study design and methodology. Cumulatively, data suggest that statin therapy, although well-tolerated by the majority of patients, may evoke a greater incidence of muscle-related side effects in chronically physically active individuals and may also exacerbate CK release and presumably the skeletal muscle damage associated with acute exercise.
Other potential mediators of the relationship between acute/chronic exercise and statin myalgia include vitamin D status, genetics, and aerobic fitness.31 Therefore, a relevant question is whether clinicians should prescribe discontinuation of statin use for several days prior to endurance events, especially if heat stress or other potential causes of rhabdomyolysis are anticipated. The latter could have been particularly important for older runners in the Boston Marathon study, who were more susceptible to experience muscle injury.29
----------------------------
Statin-associated adverse muscle symptoms may present from the patient at any time after beginning therapy with these drugs. They need to be evaluated by history, physical examination and laboratory testing when appropriate. These findings may include muscle discomfort (myalgia), muscle weakness (myopathy), tenderness to palpation, with or without muscle inflammation (myositis) and/or myonecrosis. Inflammation is usually accompanied by pain and tenderness and white cell infiltration in the muscle tissue. Myonecrosis may occur in a subacute form with elevations of creatine phosphokinase (CK) with or without pain. The most severe form of myonecrosis is referred to as rhabdomyolysis (rhabdo: rod-like structures, myo: muscle, and lys: dissolution). This is the acute and massive lysis of skeletal muscle cells with significant shifts in electrolytes in the extracellular fluid and release of large amounts of CK and myoglobin into the blood plasma. The latter can result in acute renal failure due to myoglobin precipitation in the kidney tubules. Rhabdomyolysis can occur without muscle pain or weakness, but muscle pain commonly precedes or accompanies this condition. Myositis may follow acute myonecrosis. Myalgia, myopathy, myositis, and myonecrosis can be different processes in their etiology, onset, and outcome. Given this confusion, we propose a new nosology for statin-associated muscle adverse events (Table 1).
The terminology in this report differs from other expert panels, particularly in the use of less ambiguous terminology, a myalgia clinical index score, and the use of neuromuscular diagnostic studies. In the first National Lipid Association Statin Task Force Report by Muscle Experts,4 the nonspecific term "myopathy," initially proposed by the American College of Cardiology/American Heart Association/National Heart, Lung, and Blood Institute Clinical Advisory Panel5 to account for the entire spectrum of muscle-related disorders in statin-treated patients, has been replaced by "statin-associated muscle adverse events." Myopathy is no longer categorized into asymptomatic and symptomatic myopathy.4 Instead, we use the more precise neuromuscular terminology of "myalgia" to describe muscle pain and "myopathy" to describe muscle weakness. Myositis is the term that describes muscle inflammation that is determined by skeletal muscle biopsy and/or magnetic resonance imaging. Inflammation of the muscles is commonly associated with muscle pain and tenderness. We maintain the grading of muscle injury or "myonecrosis" based on the magnitude of serum CK elevation, which is also termed hyperCKemia. This grading maintains the categories of mild, moderate, and severe CK elevations but uses the patient's own prestatin CK level vs an arbitrary normative range when baseline CK levels are available (Table 1). Other consensus documents proposed by the National Lipid Association in 2006 and adopted by the Canadian Working Group Consensus Conference6 used normative CK thresholds that do not adequately account for differences in age, sex, muscle mass, and other important clinical features that may predispose an individual to a statin adverse muscle event. Thus, we advocate the use of the individual's own CK levels when available. Severe CK elevations continue to be defined as >50 times the baseline concentration or upper normal range when baseline values are unavailable, which is consistent with the absolute CK concentration of 10,000 IU/L that was proposed by the Food and Drug Administration.7 The term "clinical rhabdomyolysis" thus represents a severe form of "myonecrosis with myoglobinuria and/or acute renal failure" to more precisely describe the clinical implications of severe statin toxicity. There is the cellular evidence that chemical toxicity or acute electrolyte changes can cause muscle cell dissolution without inflammatory infiltrate so that inflammation is not necessarily part of rhabdomyolysis. Muscle damage from autoimmune damage (inflammatory damage) or chemical toxicity can increase CK levels because both release muscle cell contents. Treating chronic muscle symptoms and severe myonecrosis with associated myoglobinuria and acute renal failure (clinical rhabdomyolysis) as different entities is more consistent with the clinical and pathologic picture. The relationship to statin therapy of these adverse muscle events requires different approaches for management.
Of these manifestations of statin-associated muscle adverse reports, myalgia complaints are most common, ranging from 1% to 5% in controlled clinical trials to 11% to 29% in observational cohorts.8, 9, 10, 11, 12, 13 In the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER), the largest and most recent clinical trial with a statin, 18,902 subjects were randomized (double blind) to rosuvastatin 20 mg daily or placebo.14 During an average follow-up of 17 months, muscle symptoms (pain, stiffness, or weakness) was noted in 1421 (16.0%) vs 1375 (15.4%), P = .34. In the rosuvastatin-treated group, myopathy was diagnosed in 10 patients vs 9 on placebo, and rhabdomyolysis occurred in one patient taking rosuvastatin. However, lower rates of myalgia and myopathy in clinical trials have been attributed to the near absence of formal assessment of muscle complaints with prospective questionnaires15 as well as the exclusion of patients with statin-associated muscle symptoms by history or prerandomization run-in phase. As a result, clinical trials may result in the underreporting of myalgia. However, clinical trials as well as observational studies may attribute muscle symptoms to statins that have other etiologies and therefore overestimate the strength of this relationship. In addition, many people with risk factors for myalgia are excluded from clinical trials, so the incidence in clinical trial participants is likely an underestimation of the incidence in clinical practice.
More consistent rates of muscle adverse events result from strict definitions with an objective laboratory measure. Rates of severe muscle injury with myoglobinuria are far less common, and reporting rates are dependent on the numbers of exposed individuals and duration of exposure.
Thus, major challenges in the assessment of statin-associated muscle toxicity derives from the absence of uniform and validated definitions of myalgia, and the lack of conducting routine muscular examinations and accepted tests of muscle performance (strength and endurance) to confirm myopathy. The use of CK, a naturally elaborated muscle enzyme for the diagnosis of muscle injury, is complicated by different racial normative levels (Fig. 1),16 differences in individuals who exercise intermittently vs regularly, and by the type of exercise (resistance vs endurance training) and the duration and intensity of the exercise.
In 2 retrospective analyses, most patients tolerated statins on repeat challenge. A recent retrospective cohort study that included electronic medical records and electronic chart reviews from medical practices affiliated with the Brigham and Women's Hospital and Massachusetts General Hospital, 2721 outpatients were rechallenged with a statin after discontinuation because of statin intolerance. Of these patients rechallenged with a statin, 92.2% remained on this treatment 12 months after the initial statin-related discontinuation.17 The authors suggested that most patients with statin-related clinical events are able to tolerate statins long term and that many of these events may have other causes. A retrospective electronic medical chart review of 1605 patients referred to the Cleveland Clinic Preventive Cardiology Service with documented statin intolerance to at least 2 statins were rechallenged with statin therapy. Of the statin-intolerant patients, intolerance that resulted primarily from myalgia, 72.5% were able to tolerate long-term (>6-month follow-up) therapy with a stable dosage of statin.18 Although these analyses suggest the need for rechallenge before a diagnosis of statin intolerance is assigned, the use of a standardized questionnaire, repeat analysis of abnormal laboratory values (serum CK, transaminases), and exclusion of other conditions or disorders unrelated to the statin were not ascertained.
In this report, we provide a paradigm to enhance accurate diagnosis of statin-associated muscle adverse events. There is a need for validated tools to accurately diagnose statin vs nonstatin muscle injury and to assess treatment approaches for the high-risk patient who is "intolerant" to statin therapy. Incident reports of statin-associated muscle injury may increase with the recent 2013 American College of Cardiology/American Heart Association Cholesterol Guidelines that recommend high-intensity and high-dose statins as initial therapy for the prevention of atherosclerotic cardiovascular events in high-risk individuals.1 This may be accompanied by greater rates of myonecrosis, particularly with simvastatin since in the Aggrastat to Zocor [A to Z] and the Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine [SEARCH], where a dose of 80 mg/day was used as the top dose.17, 18 Muscle-related symptoms with the greater dose of study drug was associated with discontinuation in 1.8% of simvastatin 40 mg/80 mg groups vs 1.5% of in those taking placebo followed by simvastatin 20 mg in the A to Z study. However, greater rates of adverse muscle events, including myonecrosis, were not different with high-dose vs low dose atorvastatin in the Treating to New Targets, or in the Incremental Decrease in Endpoints through Aggressive Lipid Lowering that compared atorvastatin 80 mg vs simvastatin 40 mg.19, 20, 21, 22
2014 Questions
The panel was asked to answer several specific questions for this report. The following questions were addressed by the current panel with their answers. Each answer is provided with an explanation and statements regarding both the strength and the quality of the evidence.
⋅1. Can statin-associated myalgia be reliably differentiated from myalgia associated with a placebo?
ANSWER: Yes.
STRENGTH OF RECOMMENDATION: B (Moderate).
QUALITY OF EVIDENCE: Moderate.
EXPLANATION: Myalgia refers to unexplained muscle discomfort often described as muscle aches, soreness, stiffness, or tenderness with or without a normal CK level. As previously noted, there are many causes of myalgia, and it is difficult to distinguish symptoms from any single cause without a significant investigation in each patient. There has been little scientific study of this question with regard to statins. A recent double-blind clinical trial, The Effect of STatins On Muscle Performance (STOMP; National Heart, Lung, and Blood Institute 5R01HL081893, NCT00609063) sought to determine the incidence of statin-associated muscle complaints in statin-naïve subjects by using a carefully defined study protocol and definition of statin myalgia.23 In brief, 202 subjects were randomly assigned to dosing with 80 mg of atorvastatin vs placebo for 6 months and were contacted by phone twice monthly during the study to inquire about muscle complaints via the Short-Form McGill Pain Questionnaire24 and Short-Form Brief Pain Inventory.25 These surveys measured the location and intensity of participants' muscle pain and the extent to which the muscle pain interfered with daily functioning. Study participants were considered to have developed statin-associated myalgia if ALL of the following occurred:
⋅new-onset or increased symptoms of myalgia (muscle aches, stiffness, cramping,
soreness, and tenderness) that were unassociated with recent exercise;
⋅symptoms that persisted for at least 2 weeks;
⋅symptoms that resolved within 2 weeks of stopping the study drug; and
⋅symptoms that reoccurred within 4 weeks of restarting the medication.
Using this standardized definition of study myalgia, we found that 24 (9.4%) of atorvastatin and 12 (4.6%) of placebo patients (P = .05) were classified with myalgia when symptoms were assessed every 2 weeks. The observation that some placebo patients satisfied the myalgia definition demonstrates the challenges with the use of self-reported symptoms to estimate the incidence of statin myalgia. Investigators from the STOMP study found no evidence of measured weakness in those patients with myalgia despite a doubling in CK levels (discussed under Dechallenge and Rechallenge section). It should also be noted that this small study did not reach the common definition of statistical significance that is P < .05.
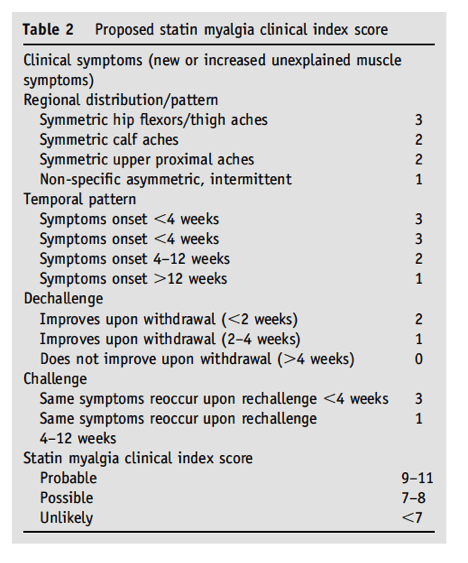
⋅2. Are there currently validated scales that can accurately diagnosis statin-associated myalgia in clinical practice?
ANSWER: No.
STRENGTH OF RECOMMENDATION: Strong.
QUALITY OF EVIDENCE: Moderate.
EXPLANATION: To date, there have been no validated scales to diagnose statin-associated myalgia. A statin-associated myalgia index is proposed based on symptoms and signs reported by participants in the aforementioned STOMP study23 and the Prediction of Muscular Risk in Observational conditions study (PRIMO).10 In this consensus of clinicians participating in this working group, we propose a quantitative myalgia score that is largely based on the findings from STOMP. This score provides various weighted values based on the distribution of the muscle complaints, temporal pattern of onset and improvement after statin withdrawal, and reoccurrence on rechallenge (Table 2). The statin myalgia clinical index score rates these symptoms as probable, possible, or unlikely related to the statin. We recognize that the statin myalgia clinical index requires validation in a prospective study that includes an independent cohort.
Clinical symptoms
A scoring system for each category of the statin myopathy clinical index is derived from data that suggest that skeletal muscle symptoms attributable to statin therapy are typically manifested as large muscle symmetric (eg, bilateral) aches (Table 2). Consequently, this symptom receives the greatest score (3 points), with bilateral aches of the smaller distal or proximal musculature receiving 2 points, and asymmetric, nonuniform symptoms receiving 1 point. For example, in the STOMP study, subjects who reported myalgia while taking atorvastatin therapy reported predominantly leg symptoms: hip flexor, quadriceps, hamstring, and/or calf aches (n = 10), quadriceps or calf cramps (n = 5), and/or quadriceps, hamstring, and/or calf fatigue (n = 6), whereas myalgic participants on placebo reported more diverse symptoms such as whole-body fatigue (n = 3), worsening of pain in previous injuries (n = 3), groin pain (n = 3), and foot cramping (n = 1). Similarly, in the PRIMO study, statin-associated muscle pain "generally affected the lower limbs."10
Temporal pattern
Studies to date indicate that symptoms of myalgia are more likely to occur within the first month of treatment. For example, in STOMP, time to symptom onset was shorter in atorvastatin-treated participants with myalgia than in placebo-treated participants with myalgia (35 ± 31 vs 61 ± 33 days; P = .045). Furthermore, in PRIMO, the median time of pain onset was 1 month. Therefore, the ranking was assigned based on the available data, suggesting that time to onset of muscle symptoms may better define true statin myalgia as opposed to myalgia associated with other diseases, deconditioning, or deficiencies.
Dechallenge and rechallenge
In STOMP, 23 atorvastatin and 14 placebo subjects reported new, unexplained muscle pain. Nineteen atorvastatin and 10 placebo subjects ultimately met the study definition for myalgia. Consequently, taking subjects off and on medication can be critical for further distinguishing the causality of muscle pain to the statin drug, and it is estimated that symptoms will be reproduced 73%-100% of the time.26 Cham et al.26 also noted that in approximately 75% of patients with statin-associated muscle side effects, discontinuation of the statin improved the symptoms, with a median time of improvement with drug discontinuation of 2 weeks and a median time of re-emergence of symptoms on rechallenge also 2 weeks. Given these data, we awarded a larger number of points based on a quicker time course of symptom abatement and return with drug dechallenge and rechallenge.
This myalgia clinical index score requires validation in a prospective trial in which patients with previous myalgia are randomized to placebo or statin and then crossed over to either statin or placebo to determine the reproducibility of symptoms. The proposed statin myalgia clinical index score represents a direction for future research initiatives.
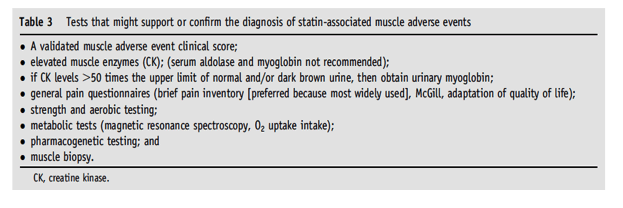
⋅4. Are there tests available to support or confirm the diagnosis of statin-associated myopathy?
ANSWER: Yes.
STRENGTH OF RECOMMENDATION: Strong.
QUALITY OF EVIDENCE: Moderate.
EXPLANATION: In these considerations, the term myopathy can be expressed by muscle weakness; however, this term is generally used to describe the entire spectrum of statin-associated muscular adverse reports. To improve the accuracy of specific skeletal muscle adverse reports, we will discuss this form of myopathy in terms of muscle weakness (not attributed to pain) and not necessarily associated with elevated CK levels (Table 3).
The diagnosis of myopathy is made by physical examination in which the individual has proximal weakness in upper and lower extremities ≤4 by Medical Research Council definition (Table 4).32 Weakness alone can be neuropathic or myopathic or both occurring simultaneously, so one must rule out neurologic compromise. This can originate in the central nervous system (eg, amyotrophic lateral sclerosis or cerebrovascular disease), in the peripheral nerves (eg, nerve root compression by spinal stenosis or diabetic neuropathy), in the neuromuscular junction (eg, myasthenia gravis), or in the vasculature (eg, polarteritis and polymyalgia rheumatic). Weakness can also result from a variety of drugs and chemicals that are listed in Table 4 as well as direct immune injury as in polymyositis or dermatomyositis. A detailed history and physical examination must be undertaken before one should simply consider statins as the causative element. We advise that individuals who experience muscle symptoms have a brief assessment of muscle strength and before initiation of treatment. Serial assessment of muscle strength is unnecessary in asymptomatic individuals and ought to occur once yearly in oligosymptomatic patients who elect to remain on statin therapy and whose CK is not >3 times the upper limit of normal. In individuals with clinically significant CK elevations and/or evidence of myopathy (Medical Research Council ≤4/5), interval muscle strength assessments throughout a dechallenge/rechallenge paradigm will enable a more confident attribution of muscle symptoms to the statin drug. Persistent myalgia and even moderate CK elevations are consistent with polymysitis. Patients with these symptoms, particularly if muscle groups are tender, need a muscle biopsy.

In endurance athletes, muscle weakness has been described as reduced exercise performance and/or prolonged recovery time after exercise. Many endurance athletes are hesitant to take statins because of the potential that this therapy will reduce their exercise performance. In these individuals, the diagnosis of myopathy may require standardized muscle testing with an isokinetic dynamometer, aerobic capacity, and respiratory exchange ratio in a fasted patient with the use of standardized procedures with a metabolic cart. A complete description of these procedures has been referenced in the methods paper to the STOMP study.33 More research into symptoms that are attributed to statins is needed. There are promising research techniques that can define muscle metabolism and functionality that may shed new light on this issue. Although not pragmatic for clinical practice these remain in the realm of highly specialized laboratories and research. These include the use of 31P magnetic resonance spectroscopy to assess phosphocreatine recovery kinetics,34 as well as the assessment of oxygen uptake kinetics during transitional exercise.35 Slowed kinetics represent an index of mitochondrial myopathies. In addition, although pharmacogenetic testing has not yet become uniform and widespread, there are many genetic variants identified that may predispose an individual to statin myalgia. For example, there are several published case studies of patients for whom statin therapy has unmasked mutations in various muscle metabolic genes related to myopathies such as McArdle disease.36 Moreover, variants in the rs4363657 single-nucleotide polymorphism located within SLCO1B1 on chromosome 12 (which is involved in regulating the hepatic uptake of statins) are strongly associated with an increased risk of statin-induced myopathy.37 Therefore, genotyping patients with a family history of statin intolerance or muscle metabolic disorders may be efficacious for diagnosing and treating statin myopathy.38, 39, 40, 41
⋅5. Are there recommendations when to obtain a muscle biopsy in patients with statin-associated muscle symptoms?
ANSWER: Yes.
STRENGTH OF RECOMMENDATION: Strong.
QUALITY OF EVIDENCE: Moderate.
EXPLANATION: Certain individuals who develop statin-associated myopathy may have an underlying histologic disorder of skeletal muscle or an inflammatory cellular infiltrate that becomes clinically manifest after taking a statin. For example, a 5-year prospective single-center study identified 52 patients with myalgia, weakness, or both, and a CK level >200 U/L (mean 1000 U/L) associated with statin therapy.42 Five patients (10%) demonstrated abnormal biopsy findings with an underlying neuromuscular disorder, whereas 47 had normal muscle biopsies. However, these CK elevations persisted after withdrawal of statin therapy, and this brings into question the causal relationship. In such studies, there have been found no consistent histologic changes that can be linked with certainty to the statin, and so these may have other coincident causes. Electromyographic changes were noted in all 5 patients with an underlying disorder but in none with normal biopsies. Routine electromyography (EMG) appears to serve as a useful screening test to identify patients with skeletal muscle pathology. However, after 6 months of follow-up, 6 patients with normal EMG and biopsy findings continued to manifest mild CK elevations (<1000 U/L). Armour and Zhou43 reported on 69 patients with statin-associated muscle adverse effects and noted only 11 of 52 patients (21%) with elevated serum CK levels. These patients were withdrawn from therapy, and had CK levels returned to normal after a mean follow-up of 18 months. Symptoms persisted in approximately 20% up to 14 months and improved in 80% by the end of follow-up. Clinicians should be aware of this group of asymptomatic patients whose condition is generally referred to as idiopathic or benign CK elevations. Because this was not a controlled study, it is not certain as to the relationship that statins may have had with the source of the disorders. Many a- or oligo-symptomatic neuromuscular conditions have been identified as causes of elevated CK levels (for review, see Silvestri and Wolfe).44 It is the position of this consensus panel that if an underlying neuromuscular disorder is identified, then it is necessary to determine whether a genuine statin-associated muscle adverse event has occurred or whether it can be explained by other mechanisms. The finding of incidental serum CK elevations in the absence of any clinically discernible change in the patient's baseline symptoms does not constitute an adverse event. Alternatively, potentiation of myalgias or weakness or escalation of CK levels in a neuromuscular patient would be considered an adverse event. However, it may not necessarily be caused by the statin. In these patients, if the symptoms are persistent and particularly if there is tenderness, we recommend a skeletal muscle biopsy to establish the diagnosis. It is unlikely that all statins have the same toxicity profiles for nerve and muscle in different neuromuscular diseases. For example, fluvastatin exhibited selective toxicity to cultured spinal motor neurons but not cortical neurons or Schwann cells.45 By contrast, atorvastatin-fed wobbler mice displayed 30% attenuation in motor neuron loss after 1 month.46 Skeletal muscle also manifests differential sensitivities to various statins in mice, with type II glycolytic fibers being more susceptible than type I oxidative fibers.47, 48
Recently, the European Federation of Neurological Societies issued a critical review on the utility of muscle biopsy in the work-up of myalgia.49 Despite the availability of only class IV studies, the consensus best practice guidelines recommended a biopsy if 1 or more of the following features are present: 1) myoglobinuria; 2) second-wind phenomenon; 3) weakness; 4) muscle hypertrophy or atrophy; 5) CK elevations (>2-3 times baseline levels or normative ranges adjusted for age, sex, and race); and/or 6) myopathic EMG.
Statin-associated muscle events do not typically cause myoglobinuria (except in rare cases of severe myotoxicity), or number 2 or 4 above.
The identification of anti-3-hydroxy-3-methylglutaryl coenzyme A reductase antibodies in the context of statin-induced necrotizing myopathy increases the need to investigate persistent CK elevations after statin withdrawal because immunosuppressive treatment can be effective, and a delay in diagnosis may decrease the likelihood of a favorable outcome.50 Persistent symptoms after statin withdrawal, particularly with elevations in serum CK levels in combination with weakness, should be investigated with a biopsy. Histopathologic diagnoses, by disclosing other treatable forms of myositis, such as polymyositis and dermatomyositis, may also lead to a more complete systemic work-up for either extra-muscular involvement or underlying neoplasm. Giant cell arteritis with polymyagia rheumatic may require a temporal artery biopsy to establish the diagnosis.
Muscle symptoms can linger beyond 14 months, and it is difficult to identify the patients who will have a prolonged recovery. CK normalization often lags behind symptom improvement, and this should not be the only indication for muscle biopsy (Table 5). Myopathic electrodiagnostic findings should lead to additional evaluation in the form of a muscle biopsy and referral to a neuromuscular specialist.
Before considering muscle biopsies in patients with moderately elevated CK levels, alternative causes for high CK levels needs to be considered first. These include recent unaccustomed or eccentric exercise, metabolic abnormalities (ie, hypothyroidism, hypoparathyroidism, vitamin D deficiency, hypophosphatemia), seizure, acute alcohol toxicity or other myotoxic drug exposure (ie, colchicine, steroids, antiretrovirals, cocaine), or intramuscular injection. We advocate that the performance of skeletal muscle biopsies be limited to individuals with severe myalgia and/or weakness in association with CK values >3 times above their untreated levels or above sex and racial norms if untreated CK levels are unavailable. Isolated asymptomatic hyperCKemia can be followed conservatively with assistance from electrodiagnostic studies. Standard practice recommends an EMG, and in those individuals with fibrillations and/or positive sharp waves in the affected muscles, a skeletal muscle biopsy is recommended to assist in correctly diagnosing the type of muscle disorder that is responsible for the CK elevation. Importantly, spontaneous activity, in the form of positive sharp waves and fibrillations, can be observed in neuropathies and radiculopathies. Therefore, thorough neurologic examinations will ultimately decide the potential utility of a muscle biopsy. It is noteworthy that no specific sets of histologic criteria have been established to classify or grade the severity of statin-related pathologic changes in human skeletal muscle. For non-specific findings, the practitioner must still contextualize the biopsy findings with clinical data to define the most appropriate clinical diagnosis.
⋅6. Can patients who are initially intolerant to one statin generally tolerate a different statin?
ANSWER: Yes.
STRENGTH OF RECOMMENDATION: B (Moderate).
QUALITY OF EVIDENCE: Moderate.
EXPLANATION: Although clinicians attempt to find the "right" statin for an individual, it is unlikely that patients who are "truly" statin intolerant will tolerate a second agent at an equivalent potency dose unless there is a clear pharmacogenomic, pharmacokinetic, or pharmacodynamic difference. As an example, the SLCO1B1 polymorphism was shown to increase the risk of myositis in simvastatin37 and cerivastatin-treated patients,51 but this pharmacokinetic interaction has not been accompanied by greater rates of skeletal muscle adverse reactions in atorvastatin or rosuvastatin-treated individuals.52, 53
Retrospective data derived from registries suggest that most patients who are intolerant to one statin because of myalgia can generally tolerate a different statin. The experience from several general practices in the Partner's HealthCare network suggest that 92% of patients can tolerate a second statin after discontinuing their initial statin due to a variety of adverse events.15 The Cleveland Clinic Preventive Cardiology Program found that of patients referred who were intolerant to two statins largely because of myalgia that 72.5% could successfully tolerate a third statin.16 Because of the use of retrospective data, nonstandard definitions of statin associated muscle adverse events and verification of abnormal laboratory values (transaminases, CK elevations after exercise) through repeat testing, these numbers certainly underestimate the clinical problem of statin intolerance but also leave the specific etiology poorly defined in such patients.
⋅7. Does the evidence base for treating statin-associated muscle symptoms or statin muscle intolerance generally consist of high-quality, randomized controlled trials with appropriate placebo or control groups?
ANSWER: No.
EXPLANATION: There are few large completed randomized studies using double-blind techniques to answer this question. Most large reports consist of observational cohorts in whom statins had been discontinued and then reintroduced at a later time. Most of the clinical trials with statins have not evaluated muscle symptoms prospectively15 or used standardized questionnaires to assess statin-associated muscle adverse events. Most often, medical records have been reviewed using search terms that do not provide context for the muscle adverse event or report whether these adverse events were reproducible on re-challenge with the use of a statin agent of equipotency.
For specific pharmacologic management, several possible approaches to the patient with statin-associated myalgia and myopathy exist including lowering the statin dosage, switching to another statin, particularly to minimize drug-drug interactions that result in elevated statin levels (see the report in this supplement from the Statin Drug Interaction Safety Panel), reducing the dosing frequency to less than daily, implementing combination lipid-lowering therapy to achieve further reductions in low-density lipoprotein cholesterol (LDL-C) levels in patients not at their LDL-C target who cannot tolerate greater-intensity statin therapy, or using a nonstatin lipid-lowering therapy or a nutraceutical approach (red yeast rice) in patients who cannot tolerate a statin at any dosage. The use of supplements, although a common practice, has not been consistently demonstrated to be beneficial. Rechallenge studies suggest that simply stopping and restarting the statin after several weeks allows for 70%-80% of patients to take a statin that may be at a reduced dosage (low-moderate intensity) or intermittently.15, 16
For patients with statin-associated muscle events, we generally suggest a written inventory of symptoms using an instrument similar to the proposed Statin Myalgia Clinical Index (Table 2) followed by a 2- to 4-week wash out and reassessment of symptoms.
Although some may consider a statin that is independent of cytochrome P450 3A4 metabolism such as pravastatin, rosuvastatin, fluvastatin, or pitavastatin, there have been no randomized studies that have compared muscle adverse events in the same trial. Some have suggested that lipophilic statins (simvastatin and lovastatin) are more likely to produce muscular effects as opposed to the more hydrophilic statins (pravastatin, rosuvastatin, and fluvastatin). Studies in animals, primarily in mice, with biopsy specimens collected before and after 8-week treatment with high-dose simvastatin (80 mg daily) and atorvastatin (40 mg daily) demonstrated that the simvastatin group alone had a greater than 50% reduction in mitochondrial DNA.54 However, the evidence supporting such a concept from human studies is extremely weak and has not been developed in a trial of appropriate design.
Alternate dosing strategies
For patients intolerant to multiple statins dosed on a daily basis, we suggest a less-than-daily dose of a different statin. The patient who is highly resistive to any statin therapy, either because of prior actual or perceived side effects, or due to fear of developing side effects, presents a growing challenge to the clinician. In this situation, we have found a once-weekly dose of a long-acting statin, such as rosuvastatin, to be an attractive option. The key to this strategy is a frank discussion with the patient regarding potential side effects, and if such side effects occur, advice to contact a physician and obtain a CK measure (if possible) before stopping the drug. Lipid levels should be checked between 4 and 12 weeks of therapy and the dose frequency increased as tolerated to achieve the desired lipid goals. The highest tolerated dose is a reasonable end point, regardless of goal attainment, because it has been demonstrated that even modest LDL-C reductions improve long-term outcomes.55
Both rosuvastatin and atorvastatin have long half-lives, making them suitable for nondaily dosing. This has the potential for lowering LDL-C levels while minimizing or preventing adverse side effects. In multiple studies of patients predominantly experiencing statin-associated myalgias, less-than-daily dosing of rosuvastatin has been tolerated in approximately three-quarters of patients who have been able to achieve an LDL-C reduction of 40%-45%.56, 57, 58, 59 In a randomized, controlled design, rosuvastatin once weekly in those with a previous statin adverse event was tolerated in 74% of the study group.60 The mean dose was 10 mg weekly with a 23% reduction in LDL-C. In addition, rosuvastatin at 5 and 10 mg twice weekly was well tolerated by 80% of patients and produced a significant reduction of LDL-C by 26%.61 Although these methods may prove beneficial, this regimen is not optimal because it lowers LDL-C much less than a daily regimen, which must be weighed particularly when treating high-risk patients. Furthermore, these dosing regimens have not been studied in terms of their effectiveness in reducing cardiovascular events.
Addition of nonstatin lipid-lowering therapy
The combination of statin plus nonstatin therapy may be used as another alternative and can even provide reductions of LDL-C similar to those seen with high-dose statin regimens. Ezetimibe may be considered in those with statin intolerance who have not achieved the LDL-C goal.62 Alternatively, a bile acid binding resin such as colesevelam may result in a 15%-19% reduction of LDL-C.63 It should be noted that there are no outcome data that have demonstrated additional cardiovascular event reduction when nonstatin LDL-C-lowering therapies are added to statin therapy.
Supplement use
Low vitamin D levels have been reported in up to 64% of individuals with statin myalgia. One symptom of vitamin D deficiency is myalgia,64 and treatment of a low vitamin D level may alleviate symptoms due to myalgia even though the improvement in symptoms may result from a mechanism unrelated to statin therapy.65 To date, however, most of the evidence is anecdotal, without prospective randomized controlled trials.
In randomized controlled trials, coenzyme Q10 (CoQ10) levels and supplementation have not been demonstrated to be helpful, although studies are ongoing.66, 67, 68 These trials have been short in duration (4-12 weeks), used lower dosages of CoQ10 than are used for the treatment of other neurologic disease such as Parkinson disease (100-400 mg/day dosage vs 1200 mg/day dosage), and involved few study participants (40-60 patients). In addition, patients who were enrolled in these trials had self-reported statin myalgia. Thus, it is not clear whether their myalgic symptoms could be reliably reproduced in the context of the study design. In the ongoing Co-Enzyme Q10 in Statin Myopathy study,69 authors will examine the effect of CoQ10 supplementation on the extent and intensity of muscle pain during treatment with simvastatin by recruiting 135 patients with a documented history of myalgia during statin treatment. The presence of statin-related myalgia will be tested in a crossover run-in trial design during, which the presence and absence of symptoms will be documented during statin and placebo treatment, respectively. Individuals who experience myalgia while taking statins, but not placebo, will be randomized to receive simvastatin 20 mg daily plus either 600 mg daily of CoQ10 or placebo. Treatment will continue for 8 weeks or until muscle symptoms are reported continuously for 1 week or become intolerable, and then subjects will crossover to the alternative treatment (CoQ10 or placebo).
Nutraceuticals, such as red yeast rice, contain monacolin K, which produces a cholesterol-lowering effect and is a form of lovastatin. However, the appreciable monacolin content may be marginal and vary between brands, and the safety of commercially available products is not known while still exposing the patient to potential statin-associated side effects.70 In a study of 43 patients with prior statin-associated myalgia, red yeast rice (2400 mg twice daily) was not superior to pravastatin (20 mg twice daily) in the development of recurrent myalgias, muscle pain, or muscle strength after 12 weeks.70 Until the Food and Drug Administration approves a formulation of red yeast rice that is suitable for the treatment of hypercholesterolemia, low-dose statin therapy still is preferable because it offers a more consistent LDL-C response at a lower cost.
In general, for patients with intolerance to their first statin, we then suggest another statin at the initial starting dose. For patients intolerant to multiple statins, we suggest a less-than-daily dose of a different statin. The patient who is highly resistive to any statin therapy, either because of previous actual or perceived side effects, or due to fear of developing side effects, presents a growing challenge to the clinician. In this situation, we have found a once weekly dose of a long-acting statin, such as rosuvastatin, to be an attractive option. The key to this strategy is a frank discussion with the patient regarding potential side effects along with prestatin initiation advice to arrange for an examination during the symptoms if possible and if intolerable to stop the drug. Lipid levels should be checked between 4 and 12 weeks of therapy, and the dose frequency increased as tolerated. The greatest tolerated dose is a reasonable end point, regardless of goal attainment, because it has been demonstrated that even modest LDL-C reductions improve long-term outcomes.55
|
| |
|
 |
 |
|
|