| |
HCV Update: New Therapies / Conference Summary, Reports, Highlights
|
| |
| |
HCV Highlights: HepDart/AASLD - Current HCV Treatments - New HCV Treatments in Development - (01/11/16)
AASLD: Merck at AASLD / HepDart 2015 - (01/14/16)
AASLD: Abbvie at AASLD / Hep Dart 2015 - (01/13/16)
AASLD: Gilead at AASLD / HepDart - Harvoni, Sofosbuvir/Velpatasvir, Sofosbuvir/Velpatasvir+GS9857 (6-8 weeks), Harvoni + Vedroprevir - (01/13/16)
AASLD: BMS at AASLD - (01/13/16)
Genotype 3 at AASLD - (01/11/16)
66th Annual Meeting of the American Association for the Study of Liver Diseases
- Boston, MA Nov 13-17 2015
HEPDART 2015: Frontiers in Drug Development for Viral Hepatitis
December 6-10 2015
Wailea, HI
I highly recommend this excellent AASLD Summary Report written by Jurgen Rockstroh, MD for NATAP:
Summary from AASLD 2015 for Hepatitis C Beyond 95% SVR cure rates: still room for improvement? - Jurgen K. Rockstroh M.D., Professor of Medicine University of Bonn, Germany - (12/29/15)
New Treatments: pangenotypic 2-3 drug regimens
from Jules: At AASLD & HepDart in Nov/Dec 2015 the latest research in HCV was reported, I have included in this report links to all the important studies, well over 100 reports including studies not just on new & current HCV therapies but also on many other key concerns like treatment around liver transplant and in patients with advanced liver disease/decompensated liver disease, cost-effectiveness, restrictions as barriers to care, costs associated with curing ALL with HCV, and of note studies on treating earlier in disease during stages F0-F2 rather than deferring until patients have cirrhosis. Of note we saw improved SVR rates to around 90% for the most difficult genotype to treat Genotype 3 with cirrhosis with daclatasvir+sofosbuvir and with Sofosbuvir+Valpatasvir, or perhaps better; see retreatment therapies below. Despite the unique medical breakthrough of the last 50 years heralding the great effectiveness of all the new HCV therapies on the market including Harvoni, Abbvie 3D and simeprevir & daclatasvir, newer highly anticipated treatments are expected to be approved in 2016. The Merck regimen of Elbasavir+Garazoprevir is composed 2 what can be considered 2nd generation in their classes, a potent protease inhibitor and a potent NS5A inhibitor, FDA approval (PDUFA date) is Jan 28 2016. The next generation Gilead regimen Sofosbuvir+Velpatasvir (GS-5816) is a pangenotypic regimen, meaning it works for all genotypes (1/2/3/4/5/6) contains a 2nd generation NS5A inhibitor (GS-5816) that is the followup NS5A to lepipasvir which is the NS5A inhibitor that is included in the combination along with sofosbuvir called Harvoni, and the expected FDA approval date is June 28, 2016. As you can see below the SVR rates are better than with Harvoni. Of note Gilead's 3rd generation treatment is a pangenotypic 3-drug regimen - Sofosbuvir+Valpatasvir+ a next generation protease inhibitor GS-9857 - you can see the link to a study of 8 weeks of treatment on this regimen reported at AASLD below. Abbvie's next generation also pangenotypic therapy is ABT-493+ABT-530, the start of the global 6-studies phase 3 in genotypes 1-6 was just announced
http://www.natap.org/2016/HCV/011116_04.htm.
ABT-493 is a next generation protease inhibitor and ABT-530 a next generation NS5A inhibitor, here are the 8 & 12 weeks treatment SVR rates below......
Merck reported early results of their next generation also pangenotypic 3-drug regimen which includes the Merck nucleotide MK-3682 (same class of drug as sofosbuvir) + a next generation NS5A inhibitor MK-8408 + their protease inhibitor Grazoprevir, see link to their study of 8 weeks treatment below. J&J is developing a 3-drug pangenotypic regimen; J&J bought out Alios nucleotides and Achillion nucleotide and next generation NS5A inhibitor, and announced in August 2015 they started a phase 1 study of simeprevir + ACH-3102 + AL-335
http://www.natap.org/2015/HCV/080315_02.htm.
Of interest to me is this 3-drug regimen achieving 100% SVR rate in patients with decompensated cirrhosis, using 3 drugs from different companies, something routine in HIV:
AASLD: SVR12 results from the Phase II, open-label IMPACT study of simeprevir (SMV) in combination with daclatasvir (DCV) and sofosbuvir (SOF) in treatment-naïve and -experienced patients with chronic HCV genotype 1/4 infection and decompensated liver disease - (11/16/15)
Of interest to me were studies on retreating patients who failed current DAAs/new HCV regimens, suggesting if initial failed treatment with current DAAs was very short in duration drug resistance mutations may not be a barrier to SVR & the new pretreatment regimen is much more effective:
AASLD: QUARTZ-I: Retreatment of HCV Genotype 1 DAA-failures With Ombitasvir/Paritaprevir/r, Dasabuvir, and Sofosbuvir - (11/17/15)
AASLD: High Rates of SVR in Patients With HCV Genotype 2 or 3 Infection Treated With Ombitasvir/Paritaprevir/r and Sofosbuvir With or Without Ribavirin - (11/23/15)
AASLD: C-SWIFT Retreatment (Part B): 12 Weeks of Elbasvir/Grazoprevir with Sofosbuvir and Ribavirin Successfully Treated G1-Infected Subjects who Failed Short-Duration All-Oral Therapy - (11/23/15)
hepDART: Retreatment of Patients Who Failed Sofosbuvir-Based Regimens with Ledipasvir/Sofosbuvir Regimens (12/22/15)
AASLD: Short-Duration Therapy With Daclatasvir/Asunaprevir/Beclabuvir Fixed-Dose Combination Plus Sofosbuvir in Patients With Chronic Hepatitis C Genotype 1 (FOURward Study) - (12/04/15)
AASLD: All-Oral Treatment With Daclatasvir Plus Sofosbuvir Plus Ribavirin for 12 or 16 Weeks in HCV Genotype 3-Infected Patients With Advanced Fibrosis or Cirrhosis: The ALLY-3+ Phase 3 Study - (11/23/15)
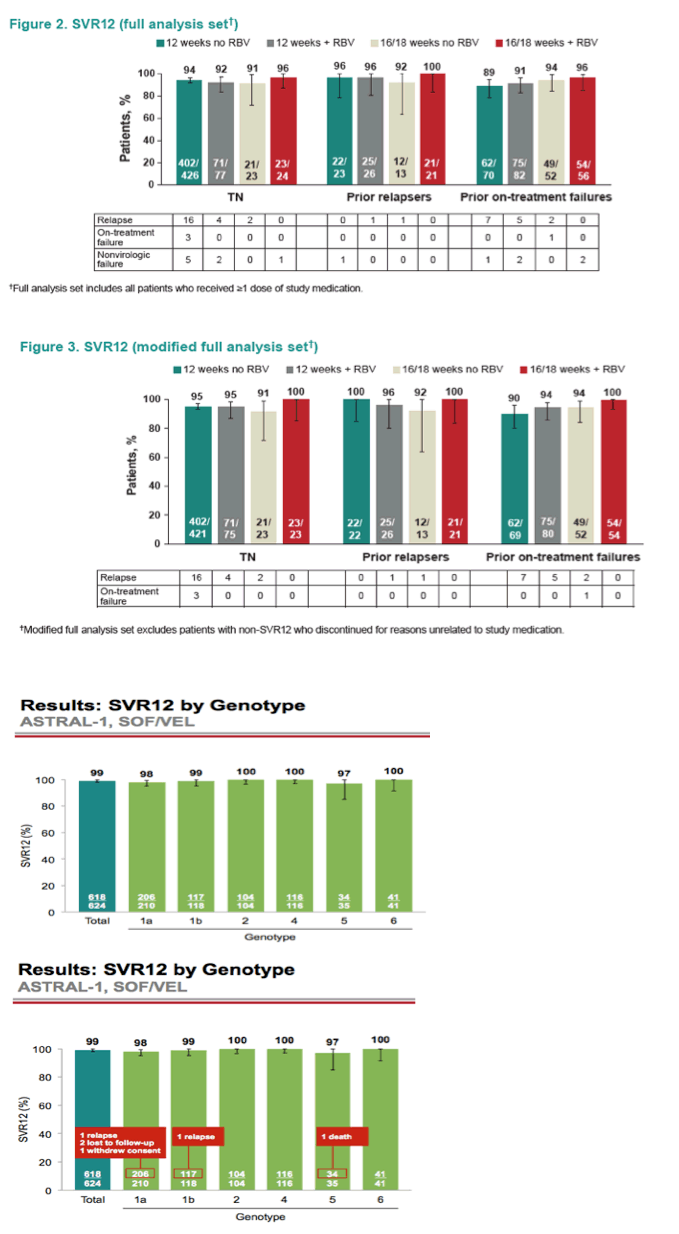
AASLD: Sofosbuvir/Velpatasvir Fixed-Dose Combination for 12 Weeks Compared to Sofosbuvir with Ribavirin for 24 Weeks in Genotype 3 HCV-Infected Patients: The Randomized Controlled Phase 3 ASTRAL-3 Study - (11/30/15)
-------------------
Of note although major large commercial insurers appear to have eliminated the fibrosis restriction that prevents anyone but cirrhosis to be treated, Medicaids have essentially retained this restriction with a very few exceptions including of course Connecticut removing restrictions due to a threatened lawsuit, and a very few Medicaids who appear to be using F2 as a cutoff for perhaps all or some patients depending on the state, and the federal government knows very well of this situation and has knowingly not resolved this problem.
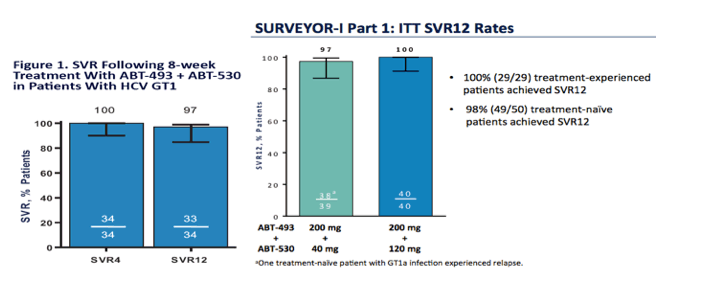
Elbasavir+Garazoprevir (Merck) Jan 28 2016 FDA approval expected
SOF/Velpatasvir(GS-5816) (Gilead) for Gt1/2/3/4/5/6 studies were reported on all these genotypes; Prescription Drug User Fee Act (PDUFA) of June 28, 2016
ABT-493+ABT-530 in phase 3
hepDART: Optimizing Outcomes in HCV Patients: Elbasvir/Grazoprevir: kidney disease/IDUs/resistance (01/04/16)
AASLD: The Combination of Elbasvir and Grazoprevir ± RBV Is Highly Effective for the Treatment of GT1a-Infected Patients - (11/17/15)
AASLD: C-EDGE CO-STAR: efficacy of grazoprevir / elbasvir Fixed Dose Combination for 12 Weeks in HCV-infected Persons Who Inject Drugs on Opioid Agonist Therapy - (11/16/15)
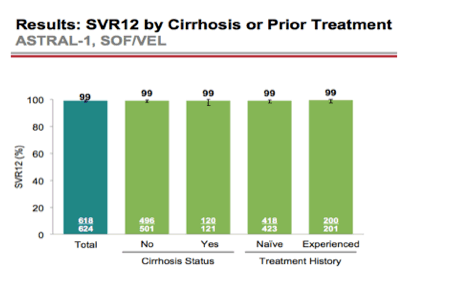
AASLD: A Phase 3 Double-Blind Placebo-Controlled Evaluation of Sofosbuvir/Velpatasvir Fixed-Dose Combination for 12 Weeks in Genotype 1, 2, 4, 5, 6 HCV-Infected Patients: Results of the ASTRAL-1 Study - (11/17/15)
AASLD: Sofosbuvir/Velpatasvir + GS-9857 for 6 or 8 Weeks in Genotype 1 or 3 HCV-Infected Patients - (11/16/15)
AASLD: 100% SVR4 in HCV Genotype 1 Non-Cirrhotic Treatment-Naïve or -Experienced Patients With the Combination of ABT-493 and ABT-530 for 8 Weeks (SURVEYOR-I)....[8 weeks] - (11/17/15)
AASLD: SURVEYOR-I: 98% - 100% SVR4 in HCV Genotype 1 Non-Cirrhotic Treatment-Naïve or Pegylated Interferon/Ribavirin Null-Responders with the Combination of the Next Generation NS3/4A Protease Inhibitor ABT-493 and NS5A Inhibitor ABT-530 - (11/16/15)
hepDART: Phase 2, Randomized, Open-Label Clinical Trials of the Efficacy and Safety of Grazoprevir and MK-3682 (NS5B Polymerase Inhibitor) with Either Elbasvir or MK-8408 (NS5A Inhibitor) in Patients with Chronic HCV GT1, 2 or 3 Infection (Part A of C-CREST 1 & 2) - (12/14/15)
|
|
| |
| |
|
|
|