| |
FDA Hepatitis Update - Approval of Zepatier for treatment of chronic hepatitis C genotypes 1 and 4
|
| |
| |
On January 28, 2016, FDA approved ZEPATIER, a fixed-dose combination product containing elbasvir, a hepatitis C virus (HCV) NS5A inhibitor, and grazoprevir, an HCV NS3/4A protease inhibitor, and is indicated with or without ribavirin for treatment of chronic HCV genotypes 1 or 4 infection in adults. The approval provides another oral treatment option for patients with genotypes 1 and 4 HCV infections without requiring use of interferon.
The safety and efficacy of Zepatier with or without ribavirin was evaluated in clinical trials of 1,373 participants with chronic HCV genotype 1 or 4 infections with and without cirrhosis.
DOSAGE AND ADMINISTRATION
Testing Prior to the Initiation of Therapy
NS5A Resistance Testing in HCV Genotype 1a-Infected Patients
Testing patients with HCV genotype 1a infection for the presence of virus with NS5A resistance-associated polymorphisms is recommended prior to initiation of treatment with ZEPATIER to determine dosage regimen and duration. In subjects receiving ZEPATIER for 12 weeks, sustained virologic response (SVR12) rates were lower in genotype 1a-infected patients with one or more baseline NS5A resistance-associated polymorphisms at amino acid positions 28, 30, 31, or 93.
Hepatic Laboratory Testing
Obtain hepatic laboratory testing prior to and during treatment with ZEPATIER.
The recommended dosage of ZEPATIER is one tablet taken orally once daily with or without food as follows in Table 1.
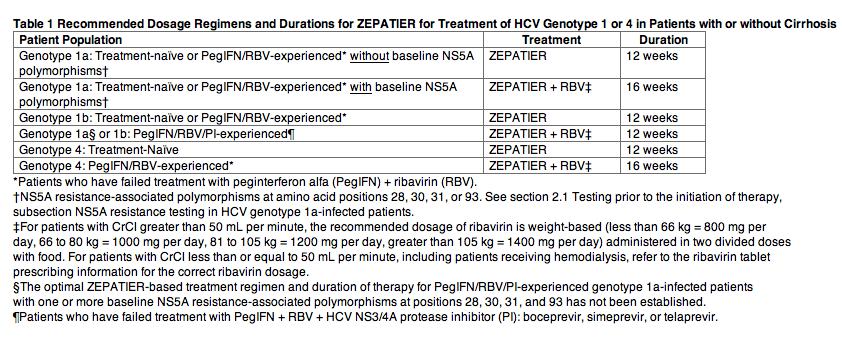
Use in Renal Impairment
No dosage adjustment of ZEPATIER is recommended in patients with any degree of renal impairment including patients on hemodialysis. Administer ZEPATIER with or without ribavirin according to the recommendations in Table 1. Refer to the ribavirin tablet prescribing information for the correct ribavirin dosage for patients with CrCl less than or equal to 50 mL per minute.
Use in Hepatic Impairment
No dosage adjustment of ZEPATIER is recommended in patients with mild hepatic impairment (Child-Pugh A). ZEPATIER is contraindicated in patients with moderate or severe hepatic impairment (Child-Pugh B or C)
Contraindications
⋅ ZEPATIER is contraindicated in patients with moderate or severe hepatic impairment (Child-Pugh B or C) due to the expected significantly increased grazoprevir plasma concentration and the increased risk of alanine aminotransferase (ALT) elevations [see Warnings and Precautions (5.1), Use in Specific Populations (8.9), and Clinical Pharmacology (12.3)].
⋅ ZEPATIER is contraindicated with organic anion transporting polypeptides 1B1/3 (OATP1B1/3) inhibitors, strong inducers of cytochrome P450 3A (CYP3A), and efavirenz [see Warnings and Precautions (5.3), Drug Interactions (7), and Clinical Pharmacology (12.3)].
⋅ If ZEPATIER is administered with ribavirin, the contraindications to ribavirin also apply to this combination regimen. Refer to the ribavirin prescribing information for a list of contraindications for ribavirin.
Table 2 lists drugs that are contraindicated with ZEPATIER.
Table 2: Drugs that are Contraindicated with ZEPATIER
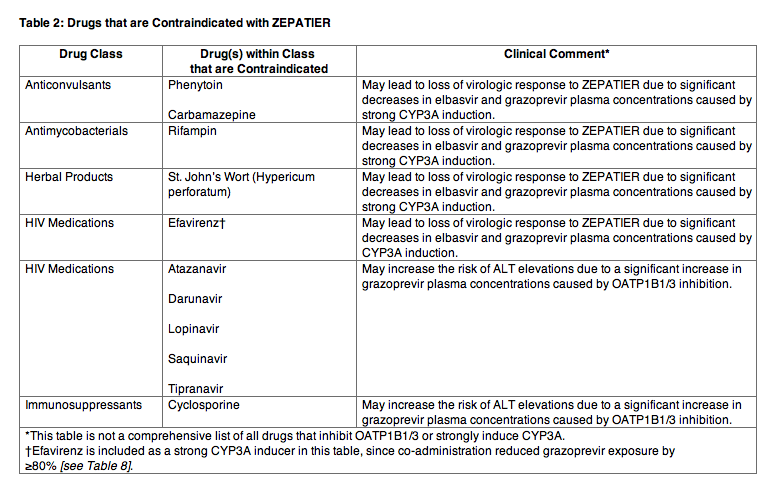
WARNINGS AND PRECAUTIONS
Increased Risk of ALT Elevations
During clinical trials with ZEPATIER with or without ribavirin, 1% of subjects experienced elevations of ALT from normal levels to greater than 5 times the upper limit of normal (ULN), generally at or after treatment week 8. ALT elevations were typically asymptomatic and most resolved with ongoing or completion of therapy. Higher rates of late ALT elevations occurred in the following subpopulations: female sex (2% [10/608]), Asian race (2% [4/164]), and age 65 years or older (2% [3/177]) [see Adverse Reactions (6.1)].
Hepatic laboratory testing should be performed prior to therapy, at treatment week 8, and as clinically indicated. For patients receiving 16 weeks of therapy, additional hepatic laboratory testing should be performed at treatment week 12.
⋅ Patients should be instructed to consult their healthcare professional without delay if they have onset of fatigue, weakness, lack of appetite, nausea and vomiting, jaundice, or discolored feces.
⋅ Consider discontinuing ZEPATIER if ALT levels remain persistently greater than 10 times the ULN.
⋅ Discontinue ZEPATIER if ALT elevation is accompanied by signs or symptoms of liver inflammation or increasing conjugated bilirubin, alkaline phosphatase, or INR.
Risks Associated with Ribavirin Combination Treatment
If ZEPATIER is administered with ribavirin, the warnings and precautions for ribavirin, including the pregnancy avoidance warning, also apply to this combination regimen. Refer to the ribavirin prescribing information for a full list of warnings and precautions for ribavirin [see Dosage and Administration (2.2)].
Risk of Adverse Reactions or Reduced Therapeutic Effect Due to Drug Interactions
The concomitant use of ZEPATIER and certain drugs may result in known or potentially significant drug interactions, some of which may lead to:
⋅ Possible clinically significant adverse reactions from greater exposure of concomitant drugs or components of ZEPATIER.
⋅ Significant decrease of elbasvir and grazoprevir plasma concentrations which may lead to reduced therapeutic effect of ZEPATIER and possible development of resistance.
ADVERSE REACTIONS
Adverse Reactions with ZEPATIER in Treatment-NaÏve Subjects
C-EDGE TN was a Phase 3 randomized, double-blind, placebo-controlled trial in 421 treatment-naÏve (TN) subjects with HCV infection who received ZEPATIER or placebo one tablet once daily for 12 weeks. Adverse reactions (all intensity) occurring in C-EDGE TN in at least 5% of subjects treated with ZEPATIER for 12 weeks are presented in Table 3. In subjects treated with ZEPATIER who reported an adverse reaction, 73% had adverse reactions of mild severity. The type and severity of adverse reactions in subjects with compensated cirrhosis were comparable to those seen in subjects without cirrhosis. No subjects treated with ZEPATIER or placebo had serious adverse reactions. The proportion of subjects treated with ZEPATIER or placebo who permanently discontinued treatment due to adverse reactions was 1% in each group.
Table 3: Adverse Reactions (All Intensity) Reported in ≥5% of Treatment-NaÏve Subjects with HCV Treated with ZEPATIER for 12 Weeks in C-EDGE TN

C-EDGE COINFECTION was a Phase 3 open-label trial in 218 treatment-naÏve HCV/HIV co-infected subjects who received ZEPATIER one tablet once daily for 12 weeks. Adverse reactions (all intensity) reported in C-EDGE COINFECTION in at least 5% of subjects treated with ZEPATIER for 12 weeks were fatigue (7%), headache (7%), nausea (5%), insomnia (5%), and diarrhea (5%). No subjects reported serious adverse reactions or discontinued treatment due to adverse reactions. No subjects switched their antiretroviral therapy regimen due to loss of plasma HIV-1 RNA suppression. Median increase in CD4+ T-cell counts of 31 cells per mm3 was observed at the end of 12 weeks of treatment.
Adverse Reactions with ZEPATIER with or without Ribavirin in Treatment-Experienced Subjects
C-EDGE TE was a Phase 3 randomized, open-label trial in treatment-experienced (TE) subjects. Adverse reactions of moderate or severe intensity reported in C-EDGE TE in at least 2% of subjects treated with ZEPATIER one tablet once daily for 12 weeks or ZEPATIER one tablet once daily with ribavirin for 16 weeks are presented in Table 4. No subjects treated with ZEPATIER without ribavirin for 12 weeks reported serious adverse reactions or discontinued treatment due to adverse reactions. The proportion of subjects treated with ZEPATIER with ribavirin for 16 weeks with serious adverse reactions was 1%. The proportion of subjects treated with ZEPATIER with ribavirin for 16 weeks who permanently discontinued treatment due to adverse reactions was 3%. The type and severity of adverse reactions in subjects with cirrhosis were comparable to those seen in subjects without cirrhosis.
Table 4: Adverse Reactions (Moderate or Severe Intensity) Reported in ≥2% of PegIFN/RBV-Experienced Subjects with HCV Treated with ZEPATIER for 12 Weeks or ZEPATIER + Ribavirin for 16 Weeks in C-EDGE TE
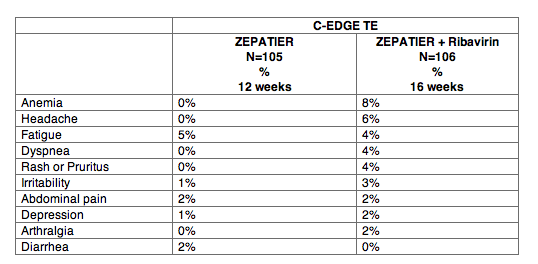
The type and severity of adverse reactions with ZEPATIER with or without ribavirin in 10 treatment-experienced subjects with HCV/HIV co-infection were comparable to those reported in subjects without HIV co-infection. Median increase in CD4+ T-cell counts of 32 cells/mm3 was observed at the end of 12 weeks of treatment with ZEPATIER alone. In subjects treated with ZEPATIER with ribavirin for 16 weeks, CD4+ T-cell counts decreased a median of 135 cells per mm3 at the end of treatment. No subjects switched their antiretroviral therapy regimen due to loss of plasma HIV-1 RNA suppression. No subject experienced an AIDS-related opportunistic infection.
C-SALVAGE was a Phase 2 open-label trial in 79 PegIFN/RBV/PI-experienced subjects. Adverse reactions of moderate or severe intensity reported in C-SALVAGE in at least 2% of subjects treated with ZEPATIER once daily with ribavirin for 12 weeks were fatigue (3%) and insomnia (3%). No subjects reported serious adverse reactions or discontinued treatment due to adverse reactions.
Adverse Reactions with ZEPATIER in Subjects with Severe Renal Impairment including Subjects on Hemodialysis
The safety of elbasvir and grazoprevir in comparison to placebo in subjects with severe renal impairment (Stage 4 or Stage 5 chronic kidney disease, including subjects on hemodialysis) and chronic hepatitis C virus infection with compensated liver disease (with or without cirrhosis) was assessed in 235 subjects (C-SURFER) [see Clinical Studies (14.4)]. The adverse reactions (all intensity) occurring in at least 5% of subjects treated with ZEPATIER for 12 weeks are presented in Table 5. In subjects treated with ZEPATIER who reported an adverse reaction, 76% had adverse reactions of mild severity. The proportion of subjects treated with ZEPATIER or placebo with serious adverse reactions was less than 1% in each treatment arm, and less than 1% and 3% of subjects, respectively, permanently discontinued treatment due to adverse reactions in each treatment arm.
Table 5: Adverse Reactions (All Intensity) Reported in ≥5% of Treatment-NaÏve or PegIFN/RBV-Experienced Subjects with Stage 4 or 5 Chronic Kidney Disease and HCV Treated with ZEPATIER for 12 Weeks in C-SURFER

Laboratory Abnormalities in Subjects Receiving ZEPATIER with or without Ribavirin
Serum ALT Elevations
During clinical trials with ZEPATIER with or without ribavirin, regardless of treatment duration, 1% (12/1599) of subjects experienced elevations of ALT from normal levels to greater than 5 times the ULN, generally at or after treatment week 8 (mean onset time 10 weeks, range 6-12 weeks). These late ALT elevations were typically asymptomatic. Most late ALT elevations resolved with ongoing therapy with ZEPATIER or after completion of therapy [see Warnings and Precautions (5.1)]. The frequency of late ALT elevations was higher in subjects with higher grazoprevir plasma concentrations [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)]. The incidence of late ALT elevations was not affected by treatment duration. Cirrhosis was not a risk factor for late ALT elevations.
Serum Bilirubin Elevations
During clinical trials with ZEPATIER with or without ribavirin, regardless of treatment duration, elevations in bilirubin at greater than 2.5 times ULN were observed in 6% of subjects receiving ZEPATIER with ribavirin compared to less than 1% in those receiving ZEPATIER alone. These bilirubin increases were predominately indirect and generally observed in association with ribavirin co-administration. Bilirubin elevations were typically not associated with serum ALT elevations.
Decreased Hemoglobin
During clinical trials with ZEPATIER with or without ribavirin, the mean change from baseline in hemoglobin levels in subjects treated with ZEPATIER for 12 weeks was –0.3 g per dL and with ZEPATIER with ribavirin for 16 weeks was approximately –2.2 g per dL. Hemoglobin declined during the first 8 weeks of treatment, remained low during the remainder of treatment, and normalized to baseline levels during follow-up. Less than 1% of subjects treated with ZEPATIER with ribavirin had hemoglobin levels decrease to less than 8.5 g per dL during treatment. No subjects treated with ZEPATIER alone had a hemoglobin level less than 8.5 g per dL.
Drug Interactions
Potential for Drug Interactions
Grazoprevir is a substrate of OATP1B1/3 transporters. Co-administration of ZEPATIER with drugs that inhibit OATP1B1/3 transporters may result in a significant increase in the plasma concentrations of grazoprevir. As such, co-administration of ZEPATIER with OATP1B1/3 inhibitors is contraindicated [see Contraindications (4), Clinical Pharmacology (12.3)], and Table 2.
Elbasvir and grazoprevir are substrates of CYP3A and P-gp, but the role of intestinal P-gp in the absorption of elbasvir and grazoprevir appears to be minimal. Co-administration of moderate or strong inducers of CYP3A with ZEPATIER may decrease elbasvir and grazoprevir plasma concentrations, leading to reduced therapeutic effect of ZEPATIER.
Co-administration of ZEPATIER with strong CYP3A inducers or efavirenz is contraindicated [see Contraindications (4), Clinical Pharmacology (12.3)], and Table 2. Co-administration of ZEPATIER with moderate CYP3A inducers is not recommended [see Warnings and Precautions (5.3), Clinical Pharmacology (12.3)], and Table 6. Co-administration of ZEPATIER with strong CYP3A inhibitors may increase elbasvir and grazoprevir concentrations.
Co-administration of ZEPATIER with certain strong CYP3A inhibitors is not recommended [see Warnings and Precautions (5.3), Clinical Pharmacology (12.3)], and Table 6.
Established and other Potentially Significant Drug Interactions
If dose adjustments of concomitant medications are made due to treatment with ZEPATIER, doses should be readjusted after administration of ZEPATIER is completed.
Table 6 provides a listing of established or potentially clinically significant drug interactions. The drug interactions described are based on studies conducted with either ZEPATIER, the components of ZEPATIER (elbasvir [EBR] and grazoprevir [GZR]) as individual agents, or are predicted drug interactions that may occur with ZEPATIER.
Table 6: Potentially Significant Drug Interactions: Alteration in Dose May Be Recommended Based on Results from Drug Interaction Studies or Predicted Interactions*
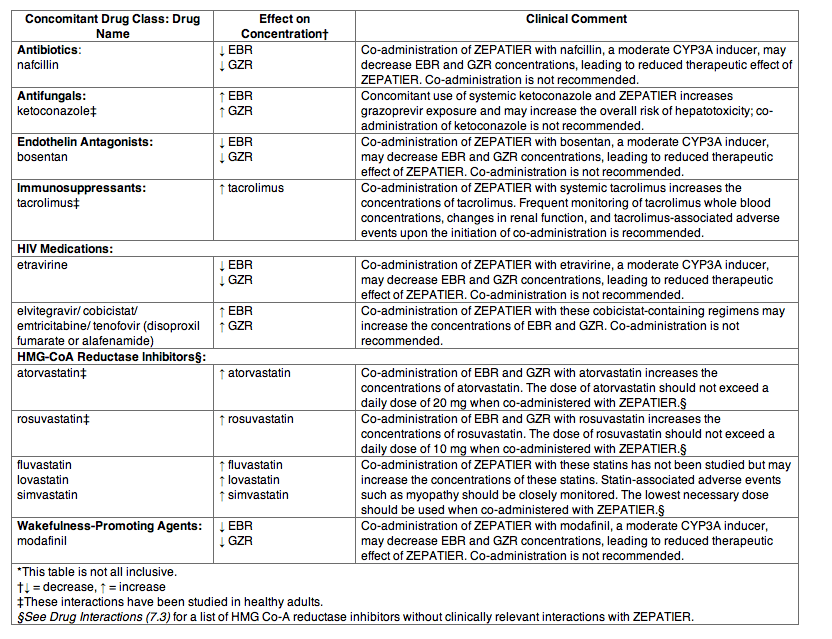
Drugs without Clinically Significant Interactions with ZEPATIER
The interaction between the components of ZEPATIER (elbasvir or grazoprevir) or ZEPATIER and the following drugs were evaluated in clinical studies, and no dose adjustments are needed when ZEPATIER is used with the following drugs individually: acid reducing agents (proton pump inhibitors, H2 blockers, antacids), buprenorphine/naloxone, digoxin, dolutegravir, methadone, mycophenolate mofetil, oral contraceptive pills, phosphate binders, pitavastatin, pravastatin, prednisone, raltegravir, ribavirin, rilpivirine, tenofovir disoproxil fumarate, and sofosbuvir [see Clinical Pharmacology (12.3)].
No clinically relevant drug-drug interaction is expected when ZEPATIER is co-administered with abacavir, emtricitabine, entecavir, and lamivudine.
Clinical Studies
Trials Conducted with ZEPATIER
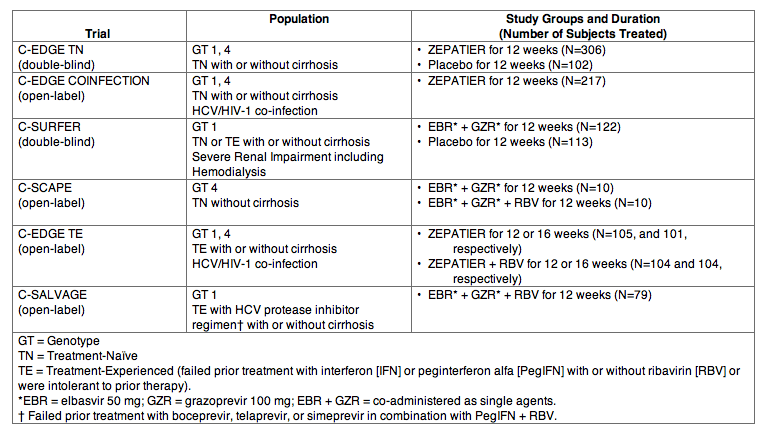
Clinical Trials in Treatment-NaÏve Subjects with Genotype 1 HCV (C-EDGE TN and C-EDGE COINFECTION)
The efficacy of ZEPATIER in treatment-naÏve subjects with genotype 1 chronic hepatitis C virus infection with or without cirrhosis was demonstrated in the C-EDGE TN and C-EDGE COINFECTION trials.
C-EDGE TN was a randomized, double-blind, placebo-controlled trial in treatment-naÏve subjects with genotype 1 or 4 infection with or without cirrhosis. Subjects were randomized in a 3:1 ratio to: ZEPATIER for 12 weeks (immediate treatment group) or placebo for 12 weeks followed by open-label treatment with ZEPATIER for 12 weeks (deferred treatment group). Among subjects with genotype 1 infection randomized to the immediate treatment group, the median age was 55 years (range: 20 to 78); 56% of the subjects were male; 61% were White; 20% were Black or African American; 8% were Hispanic or Latino; mean body mass index was 26 kg/m2; 72% had baseline HCV RNA levels greater than 800,000 IU per mL; 24% had cirrhosis; 67% had non-C/C IL28B alleles (CT or TT); and 55% had genotype 1a and 45% had genotype 1b chronic HCV infection.
C-EDGE COINFECTION was an open-label, single-arm trial in treatment-naÏve HCV/HIV-1 co-infected subjects with genotype 1 or 4 infection with or without cirrhosis. Subjects received ZEPATIER for 12 weeks. Among subjects with genotype 1 infection, the median age was 50 years (range: 21 to 71); 85% of the subjects were male; 75% were White; 19% were Black or African American; 6% were Hispanic or Latino; mean body mass index was 25 kg per m2; 59% had baseline HCV RNA levels greater than 800,000 IU per mL; 16% had cirrhosis; 65% had non-C/C IL28B alleles (CT or TT); and 76% had genotype 1a, 23% had genotype 1b, and 1% had genotype 1-Other chronic HCV infection.
Table 13 presents treatment outcomes for ZEPATIER in treatment-naÏve subjects with genotype 1 infection from C-EDGE TN (immediate treatment group) and C-EDGE COINFECTION. For treatment outcomes for ZEPATIER in genotype 4 infection, [see Clinical Studies (14.5)].
Table 13: C-EDGE TN and C-EDGE COINFECTION: SVR12 in Treatment-NaÏve Subjects with or without Cirrhosis with Genotype 1 HCV Treated with ZEPATIER for 12 Weeks
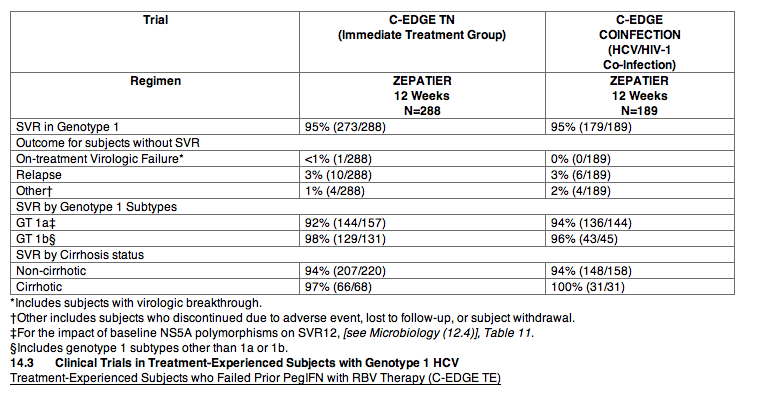
C-EDGE TE was a randomized, open-label comparative trial in subjects with genotype 1 or 4 infection, with or without cirrhosis, with or without HCV/HIV-1 co-infection, who had failed prior therapy with PegIFN + RBV therapy. Subjects were randomized in a 1:1:1:1 ratio to one of the following treatment groups: ZEPATIER for 12 weeks, ZEPATIER + RBV for 12 weeks, ZEPATIER for 16 weeks, or ZEPATIER + RBV for 16 weeks. Among subjects with genotype 1 infection, the median age was 57 years (range: 19 to 77); 64% of the subjects were male; 67% were White; 18% were Black or African American; 9% were Hispanic or Latino; mean body mass index was 28 kg/m2; 78% had baseline HCV RNA levels greater than 800,000 IU/mL; 34% had cirrhosis; 79% had non-C/C IL28B alleles (CT or TT); and 60% had genotype 1a, 39% had genotype 1b, and 1% had genotype 1-Other chronic HCV infection.
Treatment outcomes in genotype 1 subjects treated with ZEPATIER for 12 weeks or ZEPATIER with RBV for 16 weeks are presented in Table 14. Treatment outcomes with ZEPATIER with RBV for 12 weeks or without RBV for 16 weeks are not shown because these regimens are not recommended in PegIFN/RBV-experienced genotype 1 patients. For treatment outcomes for ZEPATIER in genotype 4 infection, [see Clinical Studies (14.5)].
Table 14: C-EDGE TE: SVR12 in Treatment-Experienced Subjects who Failed Prior PegIFN with RBV with or without Cirrhosis, with or without HCV/HIV-1 Co-infection with Genotype 1 HCV Treated with ZEPATIER for 12 Weeks or ZEPATIER with Ribavirin for 16 Weeks
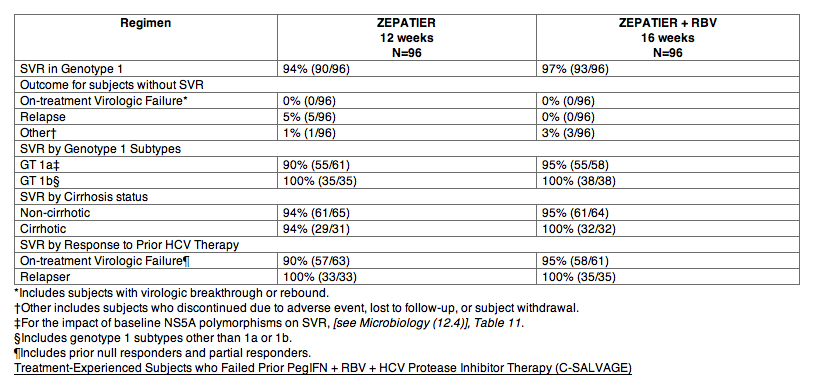
C-SALVAGE was an open-label single-arm trial in subjects with genotype 1 infection, with or without cirrhosis, who had failed prior treatment with boceprevir, simeprevir, or telaprevir in combination with pegIFN + RBV. Subjects received EBR 50 mg once daily + GZR 100 mg once daily + RBV for 12 weeks. Subjects had a median age of 55 years (range: 23 to 75); 58% of the subjects were male; 97% were White; 3% were Black or African American; 15% were Hispanic or Latino; mean body mass index was 28 kg/m2; 63% had baseline HCV RNA levels greater than 800,000 IU/mL; 43% had cirrhosis; and 97% had non-C/C IL28B alleles (CT or TT); 46% had baseline NS3 resistance-associated substitutions.
Overall SVR was achieved in 96% (76/79) of subjects receiving EBR + GZR + RBV for 12 weeks. Four percent (3/79) of subjects did not achieve SVR due to relapse. Treatment outcomes were consistent in genotype 1a and genotype 1b subjects, in subjects with different response to previous HCV therapy, and in subjects with or without cirrhosis.
Treatment outcomes were generally consistent in subjects with or without NS3 resistance-associated substitutions at baseline, although limited data are available for subjects with specific NS3 resistance-associated substitutions [see Microbiology (12.4)].
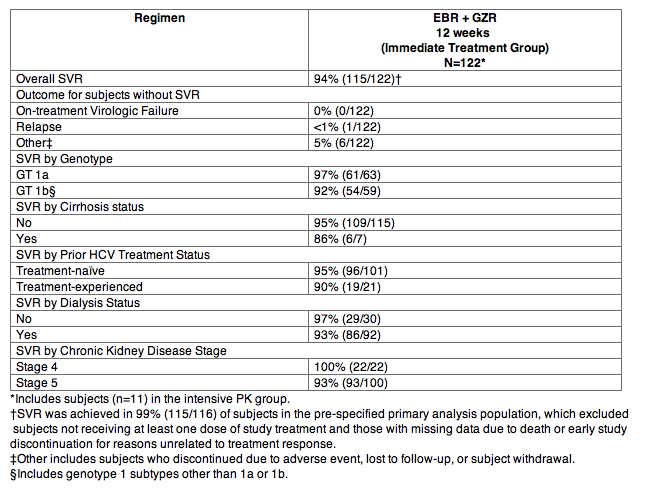
Clinical Trial in Subjects with Genotype 1 HCV and Severe Renal Impairment including Subjects on Hemodialysis (C-SURFER)
C-SURFER was a randomized, double-blind, placebo-controlled trial in subjects with genotype 1 infection, with or without cirrhosis, with chronic kidney disease (CKD) Stage 4 (eGFR 15‑29 mL/min/1.73 m2) or CKD Stage 5 (eGFR <15 mL/min/1.73 m2), including subjects on hemodialysis, who were treatment-naÏve or who had failed prior therapy with IFN or PegIFN ± RBV therapy. Subjects were randomized in a 1:1 ratio to one of the following treatment groups: EBR 50 mg once daily + GZR 100 mg once daily for 12 weeks (immediate treatment group) or placebo for 12 weeks followed by open-label treatment with EBR + GZR for 12 weeks (deferred treatment group). In addition, 11 subjects received open-label EBR + GZR for 12 weeks (intensive pharmacokinetic [PK] group). Subjects randomized to the immediate treatment group and intensive PK group had a median age of 58 years (range: 31 to 76); 75% of the subjects were male; 50% were White; 45% were Black or African American; 11% were Hispanic or Latino; 57% had baseline HCV RNA levels greater than 800,000 IU/mL; 6% had cirrhosis; and 72% had non-C/C IL28B alleles (CT or TT).
Treatment outcomes in subjects treated with ZEPATIER for 12 weeks in the pooled immediate treatment group and intensive PK group are presented in Table 15.
Table 15: C-SURFER: SVR12 in Subjects with Severe Renal Impairment including Subjects on Hemodialysis who were Treatment-NaÏve or had Failed Prior IFN or PegIFN ± RBV, with or without Cirrhosis, with Genotype 1 HCV Treated with ZEPATIER for 12 Weeks
Clinical Trials with Genotype 4 HCV
The efficacy of ZEPATIER in subjects with genotype 4 chronic HCV infection was demonstrated in C-EDGE TN, C-EDGE COINFECTION, C-EDGE TE, and C-SCAPE. C-SCAPE was a randomized, open-label trial which included treatment-naÏve subjects with genotype 4 infection without cirrhosis. Subjects were randomized in a 1:1 ratio to EBR 50 mg once daily + GZR 100 mg once daily for 12 weeks or EBR 50 mg once daily + GZR 100 mg once daily + RBV for 12 weeks. In these combined studies in subjects with genotype 4 infection, 64% were treatment-naÏve; 66% of the subjects were male; 87% were White; 10% were Black or African American; 22% had cirrhosis; and 30% had HCV/HIV-1 co-infection.
In C-SCAPE, C-EDGE TN, and C-EDGE COINFECTION trials combined, a total of 66 genotype 4 treatment-naÏve subjects received ZEPATIER or EBR + GZR for 12 weeks. In these combined trials, SVR12 among subjects treated with ZEPATIER or EBR + GZR for 12 weeks was 97% (64/66).
In C-EDGE TE, a total of 37 genotype 4 treatment-experienced subjects received a 12- or 16-week ZEPATIER with or without RBV regimen. SVR12 among randomized subjects treated with ZEPATIER + RBV for 16 weeks was 100% (8/8).
Effect of Baseline HCV Amino Acid Polymorphisms on Treatment Response in Genotype 1-Infected Subjects
Analyses using population nucleotide sequencing were conducted to explore the association between NS5A or NS3 amino acid polymorphisms and treatment response among treatment-naÏve and treatment-experienced genotype 1-infected subjects. Baseline NS5A polymorphisms at resistance-associated positions (focusing on any change from subtype reference at NS5A amino acid positions 28, 30, 31, or 93) were evaluated. Baseline NS3 polymorphisms at positions 36, 54, 55, 56, 80, 107, 122, 132, 155, 156, 158, 168, 170, or 175 were evaluated. Analyses of SVR12 rates pooled data from subjects naÏve to direct-acting antivirals and who received ZEPATIER with or without ribavirin in Phase 3 clinical trials, and censored subjects who did not achieve SVR12 for reasons unrelated to virologic failure.
Genotype 1a
In genotype 1a-infected subjects, the presence of one or more HCV NS5A amino acid polymorphisms at position M28, Q30, L31, or Y93 was associated with reduced efficacy of ZEPATIER for 12 weeks (Table 11), regardless of prior treatment history or cirrhosis status. The prevalence of polymorphisms at any of these positions in genotype 1a-infected subjects was 11% (62/561) overall, and 12% (37/309) specifically for subjects in the U.S. across Phase 2 and Phase 3 clinical trials evaluating ZEPATIER for 12 weeks or ZEPATIER plus ribavirin for 16 weeks. The prevalence of polymorphisms at these positions in genotype 1a-infected subjects was 6% (35/561) at position M28, 2% (11/561) at position Q30, 3% (15/561) at position L31, and 2% (10/561) at position Y93. Polymorphisms at NS5A position H58 were common (10%) and were not associated with reduced ZEPATIER efficacy, except for a single virologic failure subject whose virus had baseline M28V and H58D polymorphisms.
The SVR12 rates for subjects treated with ZEPATIER for 12 weeks were 88% (29/33) for subjects with M28V/T/L polymorphisms (n=29, 3, and 1, respectively), 40% (4/10) for subjects with Q30H/R/L polymorphisms (n=5, 3, and 2, respectively), 38% (5/13) for subjects with an L31M polymorphism, and 63% (5/8) for subjects with Y93C/H/N/S polymorphisms (n=3, 3, 1, and 1, respectively). Although data are limited, among genotype 1a-infected subjects with these NS5A polymorphisms who received ZEPATIER plus ribavirin for 16 weeks, six out of six subjects achieved SVR12. The specific NS5A polymorphisms observed in subjects treated with ZEPATIER plus ribavirin for 16 weeks included M28V (n=2), Q30H (n=1), L31M (n=2), or Y93C/H (n=1 each).

There are insufficient data to determine the impact of HCV NS5A amino acid polymorphisms in treatment-experienced subjects who failed prior PegIFN + RBV + HCV protease inhibitor therapy and received ZEPATIER with ribavirin.
In genotype 1a-infected subjects, the NS3 Q80K polymorphism did not impact treatment response. Polymorphisms at other NS3 resistance-associated positions were uncommon and were not associated with reduced treatment efficacy.
Genotype 1b
In genotype 1b-infected subjects treated with ZEPATIER for 12 weeks, SVR12 rates (non-virologic failure-censored) were 94% (48/51) and 99% (247/248) for those with and without one or more NS5A polymorphisms at position 28, 30, 31, or 93.
In genotype 1b-infected subjects, baseline NS3 polymorphisms did not impact treatment response.
Effect of Baseline HCV Polymorphisms on Treatment Response in Genotype 4-Infected Subjects
Phylogenetic analysis of HCV sequences from genotype 4-infected subjects (n=71) in the pooled analyses of subjects (non-virologic failure-censored) treated with regimens containing ZEPATIER or elbasvir + grazoprevir with or without ribavirin in Phase 2 and 3 clinical trials identified 4 HCV genotype 4 subtypes (4a, 4d, 4k, 4o). Most subjects were infected with either subtype 4a (42%) or 4d (51%); 1 to 2 subjects were infected with each of the other genotype 4 subtypes. Among subjects enrolled at U.S. study sites, 11/13 (85%) were infected with HCV subtype 4a. There were two subjects infected with HCV subtype 4d who experienced virologic failure with the regimen containing grazoprevir and elbasvir.
In genotype 4-infected subjects, SVR12 rates for subjects with baseline NS5A polymorphisms (any change from reference at NS5A amino acid positions 28, 30, 31, 58, and 93 by population nucleotide sequencing) were 100% (28/28) and for subjects without baseline NS5A polymorphisms were 95% (41/43).
In genotype 4-infected subjects, SVR12 rates for subjects with baseline NS3 polymorphisms (any change from reference at NS3 amino acid positions 36, 54, 55, 56, 80, 107, 122, 132, 155, 156, 158, 168, 170, and 175 by population nucleotide sequencing) were 100% (18/18) and for subjects without baseline NS3 polymorphisms were 96% (51/53).
The complete label will be posted soon at drugs@fda or dailymed.
View FDA Press Release
Richard Klein
Office of Special Health Issues
Food and Drug Administration
Kimberly Struble
Division of Antiviral Drug Products
Food and Drug Administration
Steve Morin
Office of Health and Constituent Affairs
Food and Drug Administration
|
|
| |
| |
|
|
|