| |
HCV reinfection incidence [high in Europe] and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe
|
| |
| |
Download the PDF here
"This is the largest cohort of HIV infected MSM patients followed-up longitudinally for HCV reinfections after initial HCV cure. We found a high reinfection incidence of 7.3/100py with an estimate that almost one third of patients being reinfected after 5 years. ..... Of 606 individuals who cleared HCV spontaneously or were successfully treated, 149 (24.6%) presented with a subsequent HCV reinfection. 30 out of 70 (43%) who cleared again or were successfully treated, presented with a second reinfection, 5 with a third, and one with a fourth reinfection.
These numbers highlight the failure of current prevention strategies and the need for specific measures in the HIV-infected MSM population at risk in Europe. With a high treatment uptake in this population even in the interferon era and with higher response rates to treatment in the acute phase of infection[27, reinfections are occurring most likely due to maintained risk behaviors. As new, well tolerated, but costly HCV treatments have become the standard of care for HCV therapy, there is an urgent need to develop strategies to prevent reinfection at such scale. It is essential to expand testing opportunities to identify at the earliest opportunity men in the early stages of infection to prevent onward transmission of infection through treatment and behavioral interventions. The men included in this study were all linked to care where if retained in care they are closely monitored and frequently tested for HCV reinfection using HCV RNA. Rapid access to effective treatment in conjunction with interventions to reduce high risk behaviours are then required."
--------------------
HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe
Jnl of Hepatology Oct 2016
Patrick Ingiliz, Thomas C Martin, Alison Rodger, Hans-Jürgen Stellbrink, Stefan Mauss, Christoph Boesecke, Mattias Mandorfer, Julie Bottero, Axel Baumgarten, Sanjay Bhagani, Karine Lacombe, Mark Nelson, Jürgen K Rockstroh, NEAT study group,
Abstract
Background and Aims
Moderate cure rates of acute hepatitis C virus (HCV) infections with pegylated interferon and ribavirin (PR) have been described in the last decade in men who have sex with men (MSM) who are coinfected with the human immunodeficiency virus (HIV). However, a subsequent high incidence of HCV reinfections has been reported regionally in men who both clear the infection spontaneously or who respond to treatment.
Methods
Retrospective analysis of reinfections in HIV infected MSM in eight centers from Austria, France, Germany, and the UK within the NEAT network between May 2002 and June 2014.
Results
Of 606 individuals who cleared HCV spontaneously or were successfully treated, 149 (24.6%) presented with a subsequent HCV reinfection. 30 out of 70 (43%) who cleared again or were successfully treated, presented with a second reinfection, 5 with a third, and one with a fourth reinfection. The reinfection incidence was 7.3/100 person-years (95% CI 6.2-8.6). A trend for lower incidence among individuals who had spontaneous cleared their incident infection than among individuals who were treated was found (Hazard ratio 0.62, 95% CI 0.38-1.02, p=0.06). Spontaneous clearance of reinfection was associated with ALT levels >1000 IU/ml and spontaneous clearance of a prior infection.
Conclusions
HCV reinfection is an issue of major concern in HIV-positive MSM. Prevention strategies are needed for high-risk groups to reduce morbidity and treatment costs. HIV-positive MSM with a prior HCV-infection should be tested every 3 to 6 months for reinfection, those who had achieved a reinfection every 3 months.
Lay summary
We evaluated the occurrence of HCV reinfection in HIV-positive men who have sex with men who were cured for a previous HCV infection. We found an alarming incidence of 7.3/100 person-years. Prevention measures need to address this specific subgroup of patients at high risk for HCV
Introduction
Liver disease represents a major cause of morbidity and mortality among patients infected with the human immunodeficiency virus (HIV) in the developed world[1. In the setting of effective combined antiretroviral therapy (cART) and the successful preservation of a patient’s immune function, chronic infection with hepatitis C virus (HCV) is currently the main cause for liver related mortality due to liver failure and hepatocellular carcinoma[[2], [3]].
In recent years, HCV seroconversions within European HIV cohorts have been reported among people who inject drugs (PWID) and men who have sex with men (MSM)[[4], [5]]. In the case of the MSM community, several outbreaks of acute HCV infection have been described in Western metropolitan areas over the last decade associated with high-risk sexual practices, genital ulcer disease and recreational drug use including parenteral administration[[6], [7], [8]]. Treatment uptake with interferon-based therapy has generally been high in the HIV-positive MSM population and high SVR rates have been reported as many, if not most, are treated in the acute infection phase[9. Yet despite these outcomes the epidemic has continued unabated [[5], [10]].
Several new direct-acting antivirals (DAAs) have been approved for interferon-free treatment of chronic HCV in Europe. Most of these agents are characterized by a favorable interaction profile with antiretroviral medication and sustained virological response (SVR) rates above 90% in clinical trials in the HIV/HCV coinfected population[[11], [12], [13], [14]]. Mathematical modeling predicts substantial reductions in HCV prevalence in HIV infected MSM within a decade[15 [16if the required scale-up in treatment uptake with these new compounds is achieved. Further benefits have been predicted if treatment is combined with an intervention to reduce behavioral risk, which makes the eradication of HCV an achievable goal in the HIV-HCV coinfected population in Western Europe.
In the presence of maintained risk behavior, HCV reinfections have been described in PWID and MSM who either cleared their initial infection spontaneously or were successfully treated with interferon-based therapies[[17], [18], [19], [20]]. In a recent meta-analysis of 61 studies, the five-year risk of HCV reinfection in HIV infected MSM was as high as 15% and higher than in studies on PWID[21. Two studies to date have described reinfection incidence among HIV-HCV coinfected MSM with reported rates of 8-15 per 100 person-years (py)[[19], [22]]. More recently, HCV reinfections have also been reported in phase 3 trials of DAA HCV compounds[[12], [14], [23]] nearly all of which have occurred among HIV infected MSM.
Data from London reported that individuals who spontaneously clear their acute infection may be at lower risk of future HCV reinfection when compared to those who are treated and achieve SVR. This indicates that a degree of protective immunity may develop for some patients[19. An effective immune response against HCV through multiple infections has been shown in animal models[24; however, studies among PWID have failed to consistently demonstrate a protective effect[[18], [25]].
An accurate description of the HCV epidemic including a concise observation of reinfections in specific populations will be crucial to achieve the goal of HCV eradication and to reduce costs of repeated DAA treatment. This study quantifies the rate of HCV reinfections among HIV infected MSM from seven urban European areas and investigates potential variables associated with repeat spontaneous viral clearance.
Methods
The dataset for this analysis was merged from eight centers in four countries within the NEAT (European AIDS Treatment Network) consortium: The Chelsea and Westminster Hospital and the Royal Free Hospitals, London, the St. Antoine Hospital, Paris, the Center for Infectiology, Berlin, the Center for Infectious Medicine Hamburg, the Center for HIV and Hepatogastroenterology, Duesseldorf, the University Hospital Bonn, and the Medical University of Vienna.
In all centers, the available data have been homogenized due to previous collaborations such as the NEAT Probe-C cohort.
All HIV-positive MSM from these centers with a history of a cured first HCV infection were identified with subsequent HCV PCR results followed through time to detect reinfection.
HCV cure was defined as follows:
⋅Patients with SVR defined by a negative HCV PCR at least 12 weeks after the end of an interferon-based treatment and at least 1 subsequent HCV PCR measurement.
⋅Patients with a spontaneously cleared HCV infection, defined by at least two negative HCV PCR measurements at least 24 weeks apart following HCV infection.
The following data were collected for all patients: Age, date of diagnosis of acute HCV infection, HCV genotype, date of HCV cure, whether cure was a result of treatment or spontaneous clearance, and date of last follow-up visit.
HCV RNA measurements were not standardized and depended on local operating procedures which ranged between once per year and every three months and in the case of newly developed ALT elevation.
Reinfection was defined as a detectable HCV RNA at any timepoint after cure, or within the above mentioned time frames, if a HCV geno-/subtype switch occurred. In men who had a reinfection episode, the following data was obtained at the first HCV infection and at each new infection episode: age, duration of HIV infection, date of first HCV infection, HCV genotype, HCV viral load at diagnosis, maximum alanine aminotransferase (ALT), CD4 cell count, HIV viral load, HIV treatment status. Date of HCV cure, whether cure was as a result of treatment or spontaneous clearance and date of last follow-up visit were further collected.
In patients who achieved multiple SVRs or spontaneous clearances, the time-point of subsequent reinfections and the described variables were documented.
Reinfection incidence was calculated using Kaplan-Meier survival time methods. The start of follow up was defined as the date of end of HCV treatment for individuals who were successfully treated and as the first negative HCV-RNA PCR for individuals who spontaneously cleared their infection. For individuals that underwent reinfection, the date of failure was taken as the date of newly positive HCV RNA PCR. Individuals who did not undergo reinfection were censored at the date of last negative HCV PCR available.
Comparison of reinfection incidences were calculated using logrank test and cox proportional hazards model. Variables associated with spontaneous clearance of reinfection were evaluated using logistic regression. Continuous variables were grouped (Age: 30, ALT 1000 IU/ml at initial infection and reinfection, HCV VL of 1st infection 500000 copies/ml, HIV VL undetectable at 1st infection, CD4 at first infection by brackets of 100 cells/ml, CD4 at reinfection by brackets of 100 cells/ml). All variables that had an association with a p-value <0.1 were further explored for association using multiple logistic regression. Only age and initial infection outcomes were available for regression with reinfection as the outcome. McNemar’s test was used to compare the spontaneous clearance proportions for the 1st and 2nd reinfections. All analyses were performed using Stata version 12.0.
Results
606 HIV-positive MSM with a documented cure of HCV infection between May 2002 and February 2014 were identified. SVR following treatment with pegylated interferon +/- ribavirin was achieved in 494 (81.5%) men, while 111 (18.3%) exhibited spontaneous viral clearance, and in one patient the type of cure was not documented.
During follow up until June 2014, 149 of these men (24.6%) presented with a de-novo acute HCV reinfection episode. In 95% (135/142) of cases, an ALT elevation above 41 IU/mL was observed.
The median CD4 cell count at reinfection was 533/mm3 (IQR 412-760) and 82% of reinfected patients had an HIV viral load less than 50 copies/ml at time of reinfection. The median duration of diagnosed HIV infection at HCV reinfection was 9 years (IQR 6-14). 70 patients had a documented cure or spontaneous clearance of their first reinfection of which 30 (43%) presented with a second reinfection a median of 1.8 years (IQR 1.2-2.4) after clearance of their prior infection. Five patients had a third reinfection, and in one patient a fourth reinfection occurred. Table 1 shows details of primary and reinfection genotypes, ages, SVR proportions and spontaneous clearance proportions.
Reinfection incidence
In calculating reinfection incidence, 54 patients from 3 centers were excluded due to incomplete datasets (no date for end of follow up, no date for start of follow up, incorrectly entered end of follow up date). 552 patients were therefore included in the analysis representing 1952 person-years (py) of follow-up with a median follow up time of 3.0 years (IQR 1.6-4.9, min 0.02/max 11.4 years).
The overall median follow-up time was 3.0 years (interquartile range (IQR) 1.6-4.9 years).
In total, 143 HCV reinfections occurred at a rate of 7.3/100 person-years (95% confidence interval (CI) 6.2-8.6). The median duration to reinfection was 2.0 years (IQR 1.1-3.3 years). There was a trend for higher reinfection incidence among individuals who achieved SVR following treatment for their incident infection (7.8/100 py) compared to reinfection incidence among individuals who had spontaneously cleared their incident infection (4.9/100 person-years; crude unadjusted hazard ratio for reinfection 0.62, 95%-CI 0.32-0.95, P = 0.06). Fig. 1 is the Kaplan-Meier curve showing survival from reinfection for all patients with 95% confidence intervals.
Sixty-four men either spontaneously cleared or were successfully treated for their subsequent HCV reinfection and had follow up data available representing 143 py of follow up (Median follow up 1.8 years IQR 0.9-2.8). Of these 64 men, 27 presented with a second reinfection at a median of 1.7 years (IQR 1.2-2.4) after cure of the prior infection. The second reinfection incidence rate was significantly higher than the first reinfection incidence at 18.8/100py (95% CI 12.9-27.5; hazard ratio for second reinfection 2.51, 95% CI 1.7-3.8, p<0.001).
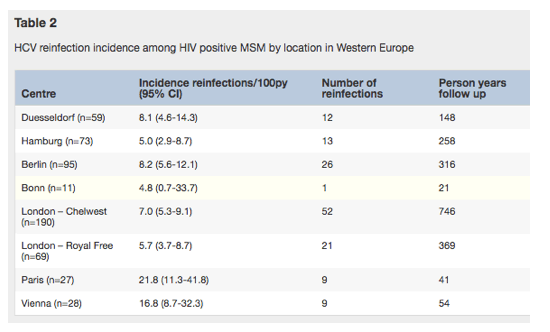
Table 2 depicts the reinfection incidence per center, with the highest being in Paris (21.8/100py, 95% CI 11.3-41.8), followed by Vienna (16.8/100py, 95%CI 8.7-32.3), Berlin (8.2/100py, 95% CI 5.6-12.1), Duesseldorf (8.1/100py, 95% CI 4.6-14.3), and London/Chelsea Westminster (7.0/100py, 95% CI 5.3-9.1). The lowest in incidence rate was seen in Hamburg (5.0/100py, 95% CI 2.9-8.7). The incidence rate decreased only slightly over time (Fig. 2).
Spontaneous clearance rates
Twenty-one of 135 patients (15.6%) spontaneously cleared their first reinfection and 7 of 22 patients (28.6%) spontaneously cleared their 2nd reinfection (p=0.43 for increase in spontaneous clearance proportion).
Men who spontaneously cleared their incident infection were less likely to present with a subsequent reinfection episode than men who had achieved SVR to their incident infection (OR 0.52, 95% CI 0.29-0.91, p=0.02). In multivariable analysis, spontaneous clearance of the first HCV infection (OR=7.47, 95%CI 1.9-29.2, p=0.004) and a maximum ALT level above 1000 IU/mL (OR=13.9, 95%CI 4.3-45.4, p<0.001) were associated with spontaneous clearance of the reinfection[26.
|
|
| |
| |
|
|
|