| |
High incidence of hepatocellular carcinoma following successful
interferon-free antiviral therapy for hepatitis C associated cirrhosis
|
| |
| |
Download the PDF here
Jnl of Hepatology Nov 2016 -
To the Editor:
We read with interest the study by Maria Reig et al. [1 on the incidence of early tumor recurrence in patients with hepatitis C virus (HCV)-related hepatocellular carcinoma (HCC) undergoing interferon (IFN)-free therapy. With a note of caution, they reported an unexpected high rate and pattern of early tumor recurrence coinciding with HCV clearance.
In fact, most of the available data evaluating the impact of HCV eradication on HCC risk is from pegylated (Peg)IFN based treatments, and patients with a previously treated HCC were seldom included due to the profile of side effects. These studies have shown that cirrhotic patients achieving a sustained virological response (SVR), with regimens containing IFN, were associated with a lower risk of HCC development and annual incidence rates of 1.2-1.4% [[2], [3]]. Nonetheless, there are some concerns regarding selection bias, as patients with more advanced disease and older age were less likely to be included in clinical trials and have access to treatment, and the main risk factors identified for HCC after HCV eradication are similar to the risk factors for worse SVR with PegIFN.
In this study by Maria Reig et al. the included patients performed at least one dynamic radiological tumor assessment after starting antiviral therapy. This methodological design was very important to prevent diagnostic bias, as in most large-scale retrospective studies it is difficult to ascertain the standard surveillance and the respective patient adherence.
With the same concern, we wish to report an increased rate of HCC incidence in patients with hepatitis C associated cirrhosis that underwent successful IFN-free antiviral therapy, at our institution.
From a group of 240 patients that were treated with IFN-free regimens for HCV, we evaluated all patients with liver cirrhosis that performed regular HCC screening after the start of antiviral therapy. Those with "non-characterized nodules" and a previous diagnosis of HCC were excluded. We included 54 patients that were treated with sofosbuvir and ledipasvir for 24 weeks in 2015. After a median follow-up of 12.0 months (IQR 9.4-12.5 months), since viral suppression, 7.4% were diagnosed with HCC. The median time for HCC development was 7.6 months (IQR 6.3-10.6 months), after HCV RNA became non-detectable. The overall characterization of the included patients is presented in Table 1.
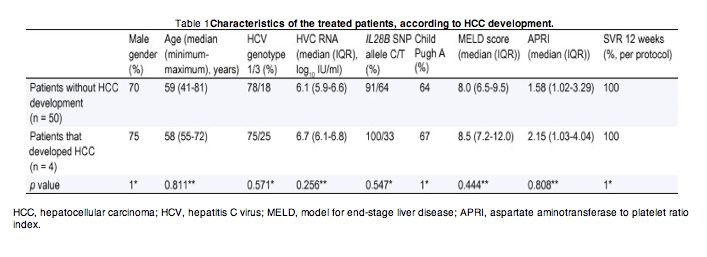
HCC, hepatocellular carcinoma; HCV, hepatitis C virus; MELD, model for end-stage liver disease; APRI, aspartate aminotransferase to platelet ratio index.
Fisher's exact test, 2-sided; A = 0.05. **Mann-Whitney U test; A = 0.05.
The de novo HCC patients were detected without specific symptoms in an ultrasonographic surveillance program and had diagnostic criteria at dynamic CT or MRI, that were validated in the multidisciplinary oncological meeting, according to European Association for the Study of the Liver (EASL) guidelines [4. All patients were referred for HCC treatment with liver transplantation and/or locoregional therapy.
The detected HCC incidence (7.4% in the first year), in cirrhotic patients achieving SVR, was higher than the previously reported for regimens containing IFN (1.2-1.4%). The affected patients had no significant differences in baseline variables that could be associated with an increased HCC risk, namely regarding age, HCV genotype, HVC viral load, IL28B single-nucleotide polymorphism, aminotransferase to platelet ratio index (APRI) score and liver function assessed by Child-Pugh or model for end-stage liver disease (MELD) score [[5], [6]]. Furthermore, there seems to be no selection bias regarding eligibility to PegIFN treatment, as most affected patients did not have contraindications for this less effective therapy.
We agree with Maria Reig et al. that a direct oncogenic effect of the antivirals is highly unlikely, but due to the coincidence with viral clearance the responsible mechanisms will probably be similar. In fact, the increased incidence comparing to PegIFN treatment suggests an important role of the immune system. In their study they performed a thorough discussion about the mechanisms that could explain the disturbance of immune cancer surveillance and the possible anti-proliferative effects of IFNs. Likewise, in chronic HCV the upregulated inflammatory status with heterogeneous activation of immune cells contributes to the control of new malignant cells growth.
Another concern is that clinical trials and other cohorts have not yet reported the same findings regarding HCC incidence, which could be related with study design, follow-up time and HCC screening implementation. Nevertheless, these results should be evaluated in larger studies, specifically addressing patients with significant fibrosis.
Authors' contributions
Helder Cardoso - conception and design of the study; acquisition of data; analysis and interpretation of data; writing of the article; final approval of the submitted version. Ana Maria Vale - conception and design of the study; acquisition of data; critical revision of the article; final approval of the submitted version. Susana Rodrigues - conception and design of the study; acquisition of data; analysis and interpretation of data, writing of the article; final approval of the submitted version. Regina Gonçalves - acquisition of data; critical revision of the article; final approval of the submitted version. Andreia Albuquerque - acquisition of data; critical revision of the article; final approval of the submitted version. Pedro Pereira - acquisition of data; critical revision of the article; final approval of the submitted version. Susana Lopes - acquisition of data; critical revision of the article; final approval of the submitted version. Marco Silva - acquisition of data; analysis and interpretation of data; critical revision of the article; final approval of the submitted version. Patricia Andrade - acquisition of data; analysis and interpretation of data; critical revision of the article; final approval of the submitted version. Rui Morais - acquisition of data; critical revision of the article; final approval of the submitted version. Rosa Coelho - acquisition of data; critical revision of the article; final approval of the submitted version. Guilherme Macedo - conception and design of the study; acquisition of data; analysis and interpretation of data; critical revision of the article; final approval of the submitted version.
|
|
| |
| |
|
|
|