| |
Does first-line antiretroviral regimen impact risk for chronic
kidney disease whatever the risk group?
|
| |
| |
Download the PDF here
ART & Kidney Disease / predictive value of the D:A:D score
-
-----------------
The Kidney Report - CROI 2016 - Christina Wyatt MD Associate Professor, Medicine/ Nephrology Icahn School of Medicine at Mount Sinai New York, NY (02/26/16)
CROI: ARTs & Mitochondrial toxicity......AZT/NNRTI induces greater adipose tissue mitochondrial toxicity than AZT/PI...... These findings support emerging data on NNRTIs contribution to thymidine-NRTI toxicity, suggesting additive MtTox as an underlying mechanism. - (04/4/16)
CROI: Mitochondrial DNA Copy Number and Neurocognitive Impairment in HIV-Infected Persons....."mtDNA associated with worse cognitive outcomes".....both HIV & ART duration associated with mtDNA damage - (03/28/16)
-----------------
Does first-line antiretroviral regimen impact risk for chronic kidney disease whatever the risk group?
"Our results confirmed, as expected, the predictive value of the D:A:D score [12] in a large population of French patients initiating ART. Moreover, we showed that when the initial risk of CKD was low according to the score, the choice of the initial regimen has little impact on CKD incidence. Although the soon available tenofovir alafenamide comes with promises of fewer renal toxicity than tenofovir [14], our results show that in patients with low risk of CKD, tenofovir remains safe for the kidney. On the contrary, in patients with a score indicating high risk, use of boosted protease inhibitors was related with higher incidences of CKD, even worsened in case of concomitant use of tenofovir. In those patients regimens avoiding both the use of tenofovir and of boosted protease inhibitors should be preferred.......In the low-risk group the treatment choice had no impact on CKD incidence....... Therefore the D:A:D score appears not only as a risk predictor tool but definitely as a clinical help for choosing the best ART in order to preserve renal function that has no equivalent.....
The complexity of scientific and reliable assessment of GFR must not let us forget what the point is in classifying renal filtration rate value for a patient. The aim of GFR determination is to estimate the risk for further renal and cardiovascular events. The precision we need to afford optimal care to our patients is not really clear. The literature seems to allow GFR to be estimated using routine equations in people living with HIV as in the general population.
In conclusion, when choosing the ideal first antiviral regimen for one given patient, clinicians should rely on the D:A:D score and avoid some drugs in high-risk patients, whereas in low-risk patients tenofovir-including regimens may be safely prescribed, with an economic benefit due to soon available tenofovir/lamivudine generic formulations.
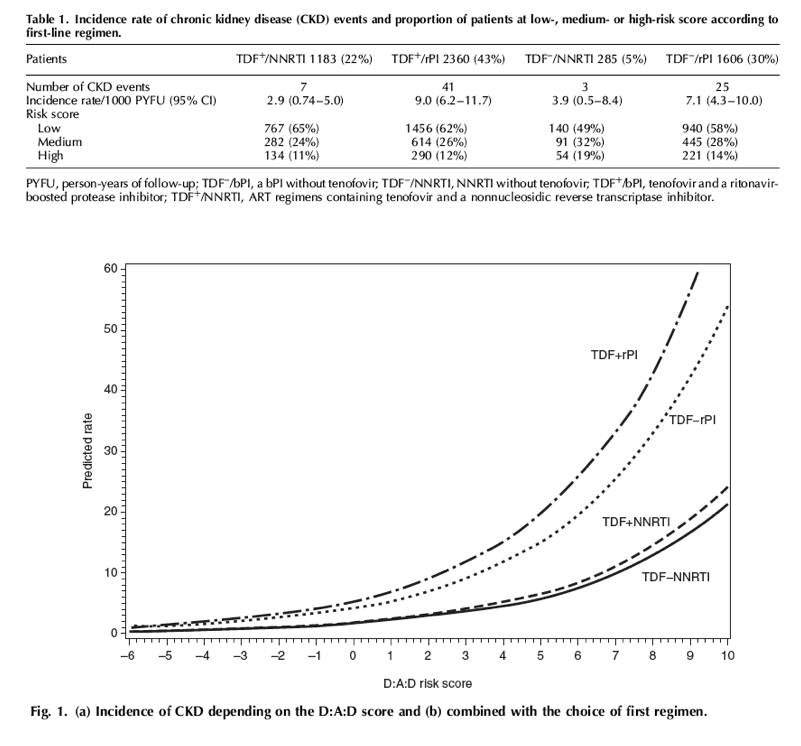
In patients with high risk of CKD (risk score ≥5) starting with a bPI provided a much higher incidence of CKD than starting with a NNRTI, worsened in case of TDF use.
Results: We included 6301 patients representing 21 936 person-years of follow-up (PYFU), median eGFR at baseline was 101 ml/min per 1.73 m2 (inter-quartile range 86; 118) and CKD incidence 9.6/1000 PYFU. Five years probabilities of CKD were 0.65, 4.6 and 15.9% in the low, medium and high-risk groups, respectively. In patients treated with a boosted protease inhibitor, incidences rates were 7.1/1000 and 9.0/1000 PYFU in the absence or presence of tenofovir, respectively, and markedly increased with increasing risk score. In the low-risk group the treatment choice had no impact on CKD incidence.
Conclusion: When choosing the ideal first antiretroviral regimen for one given patient, clinicians should rely on the D:A:D score and avoid some drugs in high-risk patients, whereas in low-risk patients classic regimens may be safely prescribed, with an economic benefit due to soon available generic formulations..
Six thousand, three hundred and one patients were included in the present work.
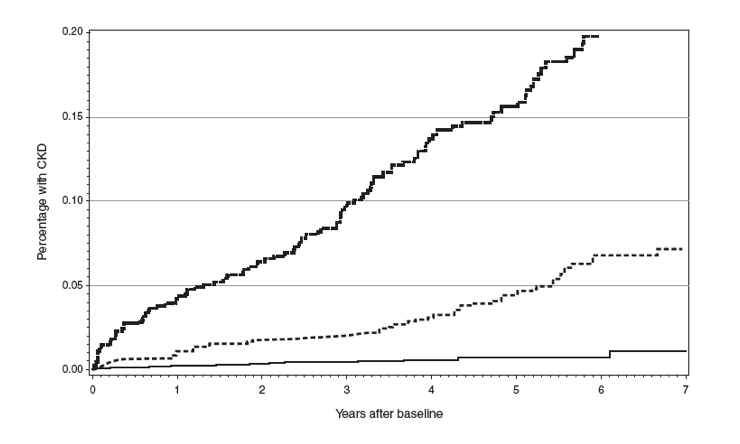
During 21 936 person-years of follow-up (PYFU), with a median follow-up of 3.1 years (IQR 1.5; 5.2) for each patient, 211 patients developed CKD (3.4%); thus the incidence of CKD was 9.6/1000 PYFU [95% confidence interval (CI) 8.3-10.9]. Patients who developed CKD were older (median 52 vs. 39 years, P<0.001), had a lower baseline eGFR (75 vs. 102 ml/min per 1.73 m2, P < 0.001), a lower CD4+ T-cell count nadir (165 vs. 266 cells/μl, P < 0.001) and a lower baseline CD4+ T-cell count (211 vs. 292 cells/μl, P < 0.001) by comparison with patients who did not develop CKD. Median D:A:D score were 5 (IQR, 2; 8) among those who developed CKD and -2 (IQR, -6; 3) among those who did not develop CKD. Using the three risk-categories, 3796 (60%) patients were at low risk and presented 22 (10%) events, 1685 (27%) patients were at medium risk and presented 67 (32%) events, and 820 (13%) patients were at high risk and presented 122 (58%) events. There was a 0.65% probability of developing CKD over the 5 years following ART initiation in the low-risk group (95% CI 0.3-0.9), increasing to 4.6% (95% CI 3.3-6.0) and 15.9% (95% CI 12.8-19.1) in the medium- and high-risk groups, respectively (Fig. 1a). The three risk groups showed a good discrimination in our population (log-rank test P < 0.001).”
-------------------
Does first-line antiretroviral regimen impact risk for chronic kidney disease whatever the risk group?
AIDS June 1 2016 Flandre, Philippe; Pugliese, Pascal; Allavena, Clotilde; Isnard Bagnis, Corinne; Cuzin, Lise; for the Dat’AIDS study Group
aINSERM, UMR-S 1136 and Sorbonne Universities, UPMC University of Paris 06, Pierre Louis Institute of Epidemiology and Public Health, Paris
bDepartment of Infectious Diseases, CHU Archet, Nice
cDepartment of Infectious Diseases, CHU Hotel Dieu, Nantes
dDepartment of Nephrology, Pitie Salpetriere Hospital APHP and Pierre et Marie Curie University, Paris
eRegional Center for HIV Care and Coordination, INSERM UMR1027, Toulouse 3 University, Toulouse, France.
Abstract
Objectives: We used the D:A:D risk score for chronic kidney disease (CKD) for patients starting antiretroviral therapy (ART) in the recent years, and investigated whether specific regimens enhanced the risk of CKD in the different risk groups.
Design: Retrospective analysis of a prospectively collected cohort of French HIV-infected patients.
Methods: Patients who started their first ART after January the 1st, 2004 with a baseline estimated glomerular filtration rate (eGFR) greater than 60 ml/min per 1.73 m2 were analyzed. CKD was defined by confirmed eGFR less than 60 ml/min per 1.73 m2. Incidence of CKD was estimated by Kaplan-Meier method, and Poisson regression models were used to quantify the relationship between CKD, exposure to the initial ART regimens and the D:A:D score.
Results: We included 6301 patients representing 21 936 person-years of follow-up (PYFU), median eGFR at baseline was 101 ml/min per 1.73 m2 (inter-quartile range 86; 118) and CKD incidence 9.6/1000 PYFU. Five years probabilities of CKD were 0.65, 4.6 and 15.9% in the low, medium and high-risk groups, respectively. In patients treated with a boosted protease inhibitor, incidences rates were 7.1/1000 and 9.0/1000 PYFU in the absence or presence of tenofovir, respectively, and markedly increased with increasing risk score. In the low-risk group the treatment choice had no impact on CKD incidence.
Conclusion: When choosing the ideal first antiretroviral regimen for one given patient, clinicians should rely on the D:A:D score and avoid some drugs in high-risk patients, whereas in low-risk patients classic regimens may be safely prescribed, with an economic benefit due to soon available generic formulations.
Introduction
Since the availability of potent antiretroviral therapy (ART), HIV-related mortality has been steadily decreasing in high-income countries [1]. Nevertheless, patients living with HIV experience increased morbidity, including chronic kidney disease (CKD) [2,3]. Some antiretroviral drugs have been associated with renal impairment [4,5] and their use requires close monitoring of the renal function. In particular, observational studies have linked tenofovir (TDF) use to a decreased estimated glomerular filtration rate (eGFR) and to increased CKD risk [2,6,7]. Some studies have also shown that concomitant use of TDF and boosted protease inhibitors (bPI) may cause greater eGFR decline than combinations with nonnucleoside reverse transcriptase inhibitors (NNRTIs) [8,9].
Identifying patients who are at an increased risk of developing CKD and who might benefit from a therapeutic or preventive intervention is of paramount importance. Risk scores have been proposed in HIV-positive individuals but have not been implemented into routine clinical practice, because of concerns about the study design [10,11]. Recently, a risk score - hereafter called the D:A:D score - for CKD based on both traditional and HIV-related risk factors has been proposed using the large D:A:D observational study [12]. The D:A:D score has been validated with two external cohorts and confirmed its promising use in clinical practice for identification of the patients at greater risk of CKD [12].
We aimed to apply this score on patients starting ART in the recent years, using a large prospective cohort of French patients living with HIV with sufficient follow-up to observe possible CKD appearance. We also investigated whether the use of TDF in combination with a bPI or a NNRTI enhanced the risk of CKD in risk groups based on the D:A:D score.
Results
Six thousand, three hundred and one patients were included in the present work. At baseline, the median age was 39 years (inter-quartile range, IQR 32; 47), the median baseline CD4+ T-cell count 289 cells/μl (IQR 175; 392) and the median viral load 4.8 log10 copies/ml (IQR 4.2; 5.3). Median eGFR at baseline was 101 ml/min per 1.73 m2 (IQR 86; 118) with 4321 (69%) patients having a baseline eGFR more than 90 ml/min per 1.73 m2. The median number of eGFR measures after ART initiation was 12 (IQR 6; 20) with a median time of 2.6 months (IQR 1.1; 3.8) between two consecutive measures.
During 21 936 person-years of follow-up (PYFU), with a median follow-up of 3.1 years (IQR 1.5; 5.2) for each patient, 211 patients developed CKD (3.4%); thus the incidence of CKD was 9.6/1000 PYFU [95% confidence interval (CI) 8.3-10.9]. Patients who developed CKD were older (median 52 vs. 39 years, P<0.001), had a lower baseline eGFR (75 vs. 102 ml/min per 1.73 m2, P < 0.001), a lower CD4+ T-cell count nadir (165 vs. 266 cells/μl, P < 0.001) and a lower baseline CD4+ T-cell count (211 vs. 292 cells/μl, P < 0.001) by comparison with patients who did not develop CKD. Median D:A:D score were 5 (IQR, 2; 8) among those who developed CKD and -2 (IQR, -6; 3) among those who did not develop CKD. Using the three risk-categories, 3796 (60%) patients were at low risk and presented 22 (10%) events, 1685 (27%) patients were at medium risk and presented 67 (32%) events, and 820 (13%) patients were at high risk and presented 122 (58%) events. There was a 0.65% probability of developing CKD over the 5 years following ART initiation in the low-risk group (95% CI 0.3-0.9), increasing to 4.6% (95% CI 3.3-6.0) and 15.9% (95% CI 12.8-19.1) in the medium- and high-risk groups, respectively (Fig. 1a). The three risk groups showed a good discrimination in our population (log-rank test P < 0.001).
Overall, 5434 patients initiated one of the four investigated ART regimens, 1183 patients starting with TDF+/NNRTI, 2360 with TDF+/rPI, 285 with TDF-/NNRTI, and 1606 with TDF-/rPI (Table 1). Among the 211 events occurring during their follow-up, 76 (36%) occurred during the original ART regimen of whom 41 and 25 in patients receiving a bPI with and without TDF, respectively (Table 1). Incidence rates, computed in the Poisson regression analysis, were significantly higher in patients receiving bPI-containing regimen, especially when associated with TDF, rather than with a NNRTI-containing regimen. Of note, 19% of patients receiving an ART regimen with a NNRTI and without TDF were in the high-risk group compared with around 12% in the three other groups. In patients receiving a bPI, incidence rates were markedly increased with increasing values of the risk score (Fig. 1b). Patients in the low-risk group (risk score < 0) had almost similar incidence rates whatever the initial ART regimen. In patients with high risk of CKD (risk score ≥5) starting with a bPI provided a much higher incidence of CKD than starting with a NNRTI, worsened in case of TDF use.
Discussion
Our results confirmed, as expected, the predictive value of the D:A:D score [12] in a large population of French patients initiating ART. Moreover, we showed that when the initial risk of CKD was low according to the score, the choice of the initial regimen has little impact on CKD incidence. Although the soon available tenofovir alafenamide comes with promises of fewer renal toxicity than tenofovir [14], our results show that in patients with low risk of CKD, tenofovir remains safe for the kidney. On the contrary, in patients with a score indicating high risk, use of boosted protease inhibitors was related with higher incidences of CKD, even worsened in case of concomitant use of tenofovir. In those patients regimens avoiding both the use of tenofovir and of boosted protease inhibitors should be preferred. Therefore the D:A:D score appears not only as a risk predictor tool but definitely as a clinical help for choosing the best ART in order to preserve renal function that has no equivalent.
The strength of our study is the large population with sufficient follow-up, seeking care in different settings in France, including oversea territories. Nonetheless, our study has limits. The D:A:D score was validated against CKD defined by Cockcroft-Gault estimations of GFR, whereas we use MDRD estimations because our available data did not allow the use of Cockcroft-Gault formula nor CKD-EPI equation, which is recommended by the latest guidelines of the Infectious Diseases Society of America [15]. CKD EPI, which is indeed recommended in the IDSA guidelines, is usually not an option in retrospective studies due to non-IDMS-traceable creatinine measurements, as in our study. Very few studies indeed documented the optimal GFR estimation equation in people living with HIV by comparison to the gold standard measurements. Most studies compared different equations without using a gold standard measurement which really does not add any information with regard to the validity of the result contrary to what the authors usually state [16,17]. The largest study was conducted by Inker et al.[18]. The authors evaluated the performance of the MDRD and CKD-EPI (Chronic Kidney Disease-Epidemiology Collaboration) creatinine 2009, CKD-EPI cystatin C 2012, and CKD-EPI creatinine-cystatin C 2012 GFR estimating equations compared with GFR measured using plasma clearance of iohexol in 200 HIV-positive patients on stable ART. Creatinine and cystatin C assays were standardized to certified reference materials. Only the creatinine-cystatin C equation was significantly more accurate than the cystatin C equation, all others exhibiting same performances. It has been definitely demonstrated that Cockcroft and Gault formulae performs worse than MDRD and CKD-EPI in the general population and also in people living with HIV. The best strategy, which is actually referred to in all nephrology guidelines for patients below 60 ml/min per 1.73 m2, remains MDRD equation [19]. The complexity of scientific and reliable assessment of GFR must not let us forget what the point is in classifying renal filtration rate value for a patient. The aim of GFR determination is to estimate the risk for further renal and cardiovascular events. The precision we need to afford optimal care to our patients is not really clear. The literature seems to allow GFR to be estimated using routine equations in people living with HIV as in the general population.
In conclusion, when choosing the ideal first antiviral regimen for one given patient, clinicians should rely on the D:A:D score and avoid some drugs in high-risk patients, whereas in low-risk patients tenofovir-including regimens may be safely prescribed, with an economic benefit due to soon available tenofovir/lamivudine generic formulations.
Methods
Patients
Information was collected from 12 large HIV reference centers in France. These hospitals maintain prospective databases of all HIV-1 infected patients who seek care in the centers and provide written consent [13]. The data collection has been approved by the French National Commission on Informatics and Liberty (CNIL). This system allows use of the databases with minimal delay, limited to automatic and manual quality controls performed before any analysis. For the purpose of this study, we selected all patients that: started their first antiretroviral regimen after 1 January 2004; had at least one serum creatinine measurement within 6 months before initiation of ART; had at least two measurements after initiation; and with baseline eGFR greater than 60 ml/min per 1.73 m2. Baseline eGFR was defined from the last serum creatinine measurement within 6 months before ART initiation. eGFR values were calculated using the Modification of Diet and Renal Disease (MDRD) equation.
Endpoints and statistical methods
CKD was defined as a confirmed (3 months apart) decrease in eGFR to less than 60 ml/min per 1.73 m2. Time to CKD was the time of first eGFR value at least 60 ml/min per 1.73 m2. The D:A:D score was calculated at baseline for each patient of our selected population. The following variables were included in the score: HIV exposure (+2 if intravenous drug user; 0 otherwise), hepatitis C co-infection (+1 if positive; 0 otherwise), age (0 if ≤35 years; +4 if > 35 to ≤50 years; +7 if >50 to ≤60 years; +10 if >60 years), baseline eGFR (-6 if >90 ml/min per 1.73 m2; 0 otherwise), sex (+1 for female; 0 otherwise), nadir CD4+ cell count (-1 if > 200 cells/μl; 0 otherwise), hypertension (+1 if yes; 0 otherwise), prior cardiovascular disease (+1 if yes; 0 otherwise) and diabetes (+2 if yes; 0 otherwise). Three risk groups were defined as low (risk score <0), medium (risk score 0-4), and high risk (risk score ≥5). Kaplan-Meier method was used to determine the probability of CKD during the follow-up for the three risk groups.
A second analysis was restricted to patients with CKD occurring during initial ART regimens. Patients who modified their first regimen were then censored at the time of modification. Poisson regression models were used to quantify the relationship between CKD, exposure to the initial ART regimens and the D:A:D score. In this analysis we only focused on four mutually exclusive ART regimens containing tenofovir and a ritonavir boosted protease inhibitor (TDF+/bPI), tenofovir and a nonnucleosidic reverse transcriptase inhibitor (TDF+/NNRTI), a bPI without tenofovir (TDF-/bPI), or a NNRTI without tenofovir (TDF-/NNRTI). Regression models were not adjusted because the main predictive factors of CKD are already included in the risk score. All analyses were done with SAS (version 9.3; SAS Institute Inc., Cary, North Carolina, USA).
|
|
| |
| |
|
|
|