 |
 |
 |
| |
Vorinostat, HCQ, Maraviroc Do Not Delay Time to Rebound After Interruption
|
| |
| |
21st International AIDS Conference (AIDS 2016), July 18-22, 2016, Durban, South Africa
Mark Mascolini
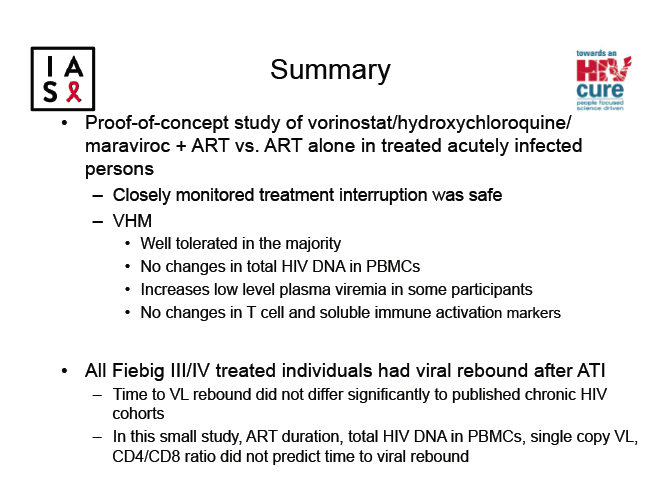
Adding vorinostat, hydroxychloroquine (HCQ), and maraviroc to antiretroviral therapy did not delay time to rebound after interruption of treatment begun during acute HIV infection, according to results of a randomized trial [1]. Considering the failure of this three-drug cure combination, the researchers concluded that "alternative strategies to reduce or eliminate HIV reservoirs are needed."
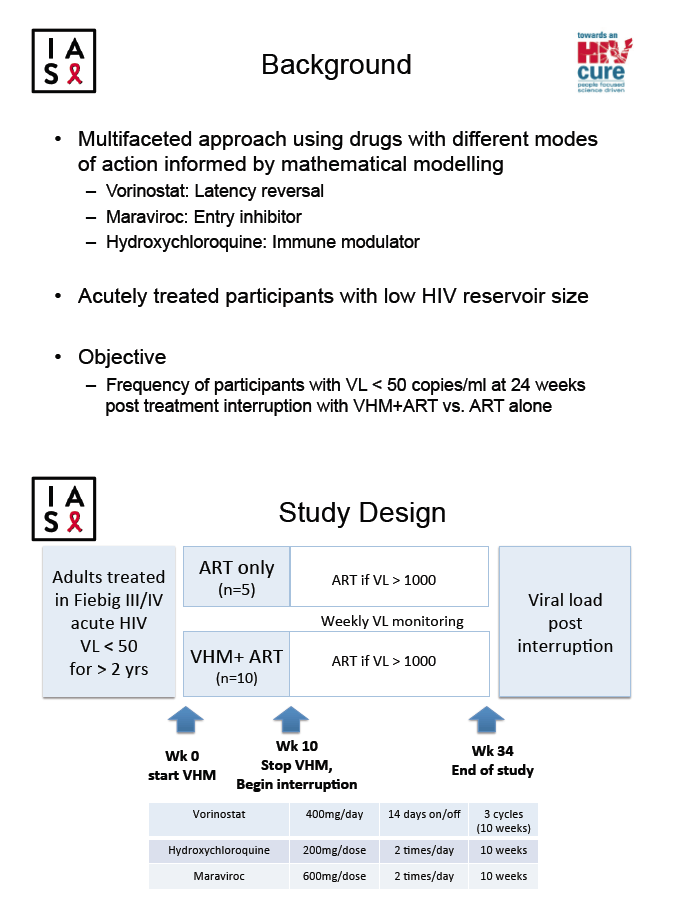
HIV reservoirs formed in the earliest days of HIV infection pose a daunting challenge to HIV cure strategies. The SEARCH 019 study group assessed the reservoir-limiting potential of antiretroviral therapy (ART) plus three agents (VHM): vorinostat (an HIV latency-reversing agent), hydroxychloroquine (an immune modulator), and maraviroc (an HIV entry inhibitor) [2]. The study involved people with acute HIV infection and thus smaller HIV reservoirs. The SEARCH 019 group aimed to determine proportions of study participants who maintained a viral load below 50 copies 24 weeks after interrupting treatment with VHM plus ART versus ART alone.
Researchers recruited adults in Thailand who began ART in Fiebig stage 3 or 4 acute HIV infection and kept their viral load below 50 copies for more than 2 years. The investigators randomized 5 participants to continue ART for 10 weeks and 10 participants to continue ART and add VHM for 10 weeks at doses 400 mg/day, 14 days on/off, for vorinostat; 200 mg twice daily for hydroxychloroquine; and 600 mg twice daily for maraviroc. At week 10 all treatment stopped. The investigators monitored viral load weekly and resumed ART at a viral load above 1000 copies.
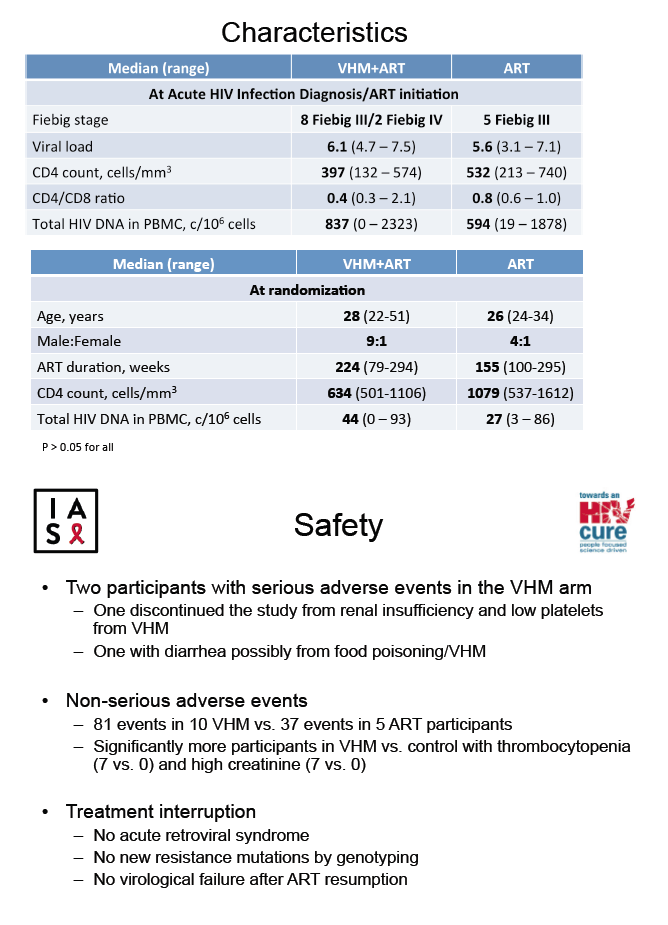
Median viral load at HIV diagnosis stood at 6.1 and 5.6 log10 copies/mL in the VHM group and the control group. Respective median CD4 counts were 397 and 532, and HIV DNA in peripheral blood mononuclear cells (PBMCs) 837 and 594 copies/million cells. At randomization in the VHM and control groups, median ages were 28 and 26, ART duration 224 and 155 weeks, male:female proportions 9:1 and 4:1, CD4 count 634 and 1079, and HIV DNA in PBMCs 44 and 27 copies/million cells.
Two people in the VHM arm had serious adverse events--one withdrew because of renal insufficiency and low platelets and one had diarrhea possibly caused by food poisoning. Significantly more people assigned to VHM plus ART than to ART alone had thrombocytopenia (7 versus 0) and high creatinine (7 versus 0). Acute retroviral syndrome did not follow treatment interruption in any participant, and all participants regained an undetectable viral load when ART resumed after treatment interruption.
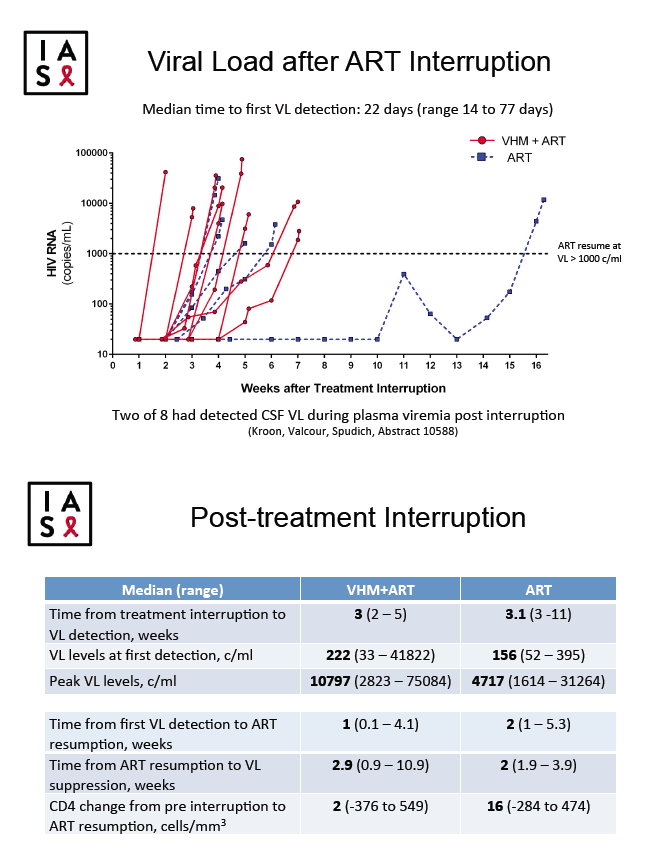
Time from ART interruption to first detectable viral load ranged from 14 to 77 days (median 22 days) and did not differ significantly between the VHM group and the ART-only group: median time to detection measured 3 weeks in the VHM group and 3.1 weeks in the control group. Viral load rebounded within 7 weeks of interruption in all but one person, a participant in the ART-only group whose viral load remained below 1000 copies for 15 weeks then rebounded. Time from resumed ART to viral suppression was also similar in the two study arms, median 2.9 weeks in the VHM group and 2.0 weeks in the ART-only group. Detectable low-level viremia increased in some participants during VHM treatment.
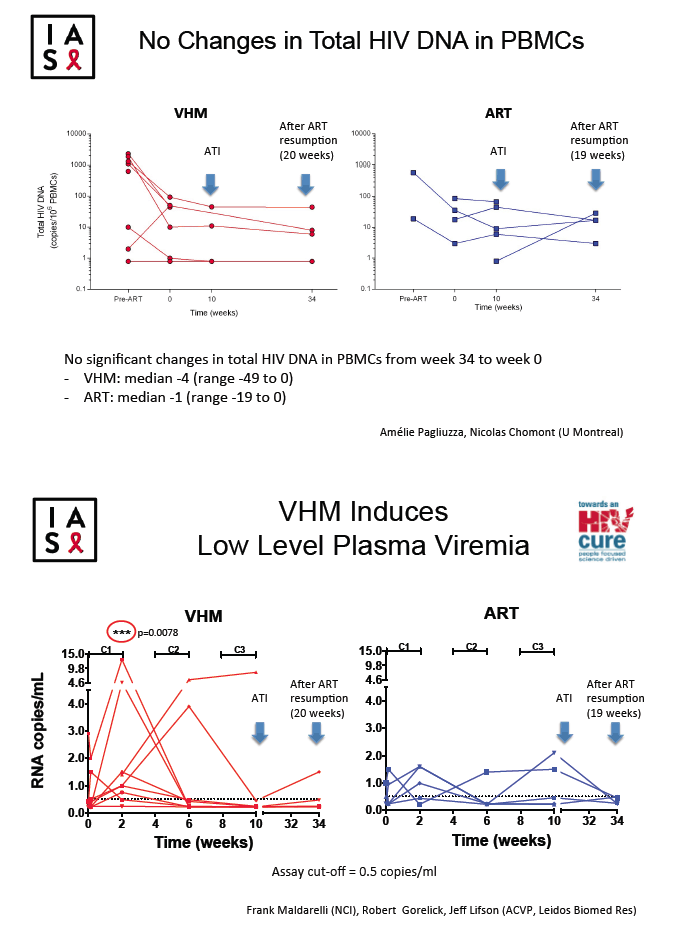
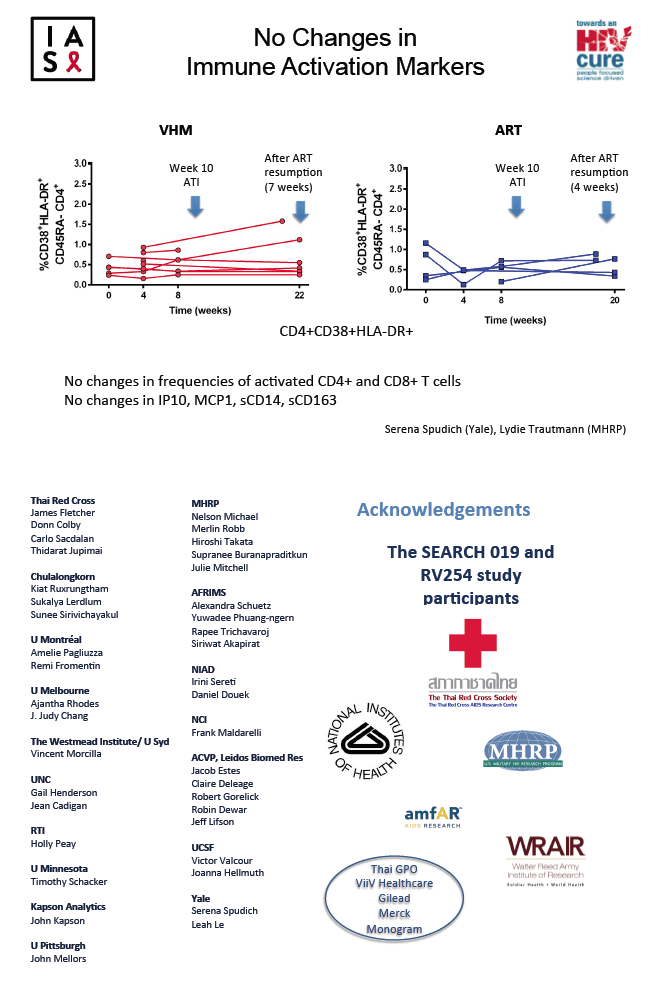
Total HIV DNA in PBMCs hardly changed in either group through 34 weeks on and off treatment, declining 4 copies/million cells in the VHM group (range -49 to 0) and 1 copy/million cells in the ART-only group (range -19 to 0). Frequency of activated CD4 or CD8 cells did not change during VHM treatment or during the treatment interruption; nor did levels of immune activation markers IP10, MCP1, sCD14, or sCD163.
The researchers noted that time to viral rebound in VHM participants did not differ significantly from rebound time in published studies of cohorts with chronic HIV infection. In this study, ART duration, total HIV DNA in PBMCs, single-copy viral load, or CD4/CD8 ratio did not predict time to viral rebound. Study results underline the difficulty of draining HIV reservoirs even in people who start ART during acute infection.
References
1. Kroon E, Ananworanich J, Eubanks K, et al. Effect of vorinostat, hydroxychloroquine and maraviroc combination therapy on viremia following treatment interruption in individuals initiating ART during acute HIV infection. 21st International AIDS Conference (AIDS 2016). July 18-22, 2016. Durban, South Africa. Abstract TUAX0101LB.
2. ClinicalTrials.gov. Efficacy of VHM after treatment interruption in subjects initiating ART during acute HIV infection. ClinicalTrials.gov identifier NCT02475915. https://clinicaltrials.gov/ct2/show/NCT02475915

|
| |
|
 |
 |
|
|