 |
 |
 |
| |
Pharmacokinetics of Dolutegravir After Switching to Abacavir/Dolutegravir/ Lamivudine From an Efavirenz-Based Regimen: A PK Sub-Study From STRIIVING
|
| |
| |
Reported by Jules Levin
17th International Workshop on Clinical Pharmacology of HIV & Hepatitis Therapy; June 8-10, 2016; Washington, DC
Joss de Wet,1 Edwin DeJesus,2 Louis Sloan,3 Justin Koteff,4 Clare Brennan,4 Kimberly Adkison,4 Deanna Merrill,5 Brian Wynne,6 Mark Shaefer,4 Michael Aboud 7
1University of British Columbia, Vancouver, BC, Canada; 2Orlando Immunology Center, Orlando, FL, USA; 3North Texas Infectious Diseases Consultants, Dallas, TX, USA; 4ViiV Healthcare, Research Triangle Park, NC, USA; 5ViiV Healthcare, Castle Rock, Colorado, USA; 6ViiV Healthcare, Collegeville, PA, USA; 7ViiV Healthcare, London, UK

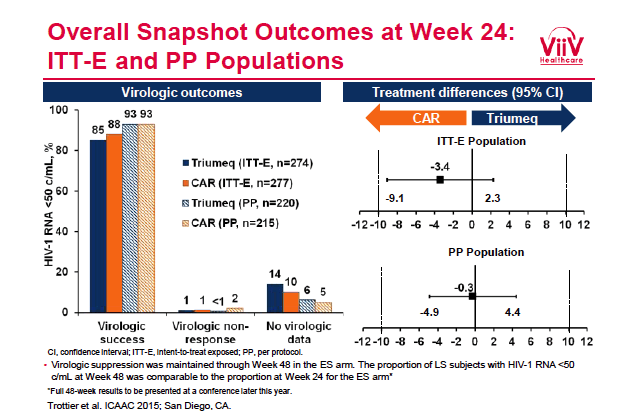
ICAAC/2015: Switching to Abacavir/Dolutegravir/Lamivudine Fixed Dose Combination (ABC/DTG/3TC FDC) from a PI, INI or NNRTI Based Regimen Maintains HIV Suppression - (09/21/15)
|
| |
|
 |
 |
|
|