 |
 |
 |
| |
Open-Label, Drug-Drug Interaction Study between Second-generation HIV-1 Maturation Inhibitor BMS-955176 and Tenofovir in Healthy Subjects
|
| |
| |
Proof-of-concept study supports BMS-955176 as a viable compound that overcomes limitations seen in studies of earlier maturation inhibitor candidates; Phase IIb studies for BMS-955176 will begin in second quarter of 2015
BMS-955176: Antiviral Activity and Safety of a Second-Generation HIV-1 Maturation Inhibitor.....
http://www.natap.org/2015/CROI/croi_36.htm
Second-Generation HIV-1 Maturation Inhibitor BMS-955176: Antiviral Activity and Safety with Atazanavir ± Ritonavir.....
http://www.natap.org/2015/IAS/IAS_25.htm
Second-generation HIV-1 Maturation Inhibitor BMS-955176: Overall Antiviral Activity and Safety Results from the Phase IIa Proof-of-Concept Study (AI468002)......
http://www.natap.org/2015/EACS/EACS_09.htm
Attachment Inhibitor Prodrug BMS-663068 in Antiretroviral-Experienced Subjects: Week 48 Analysis.....
http://www.natap.org/2015/CROI/croi_38.htm
Bristol-Myers Squibb to Sell its HIV R&D Portfolio to ViiV Healthcare.....
http://www.natap.org/2015/newsUpdates/122115_01.htm
Reported by Jules Levin
17th International Workshop on Clinical Pharmacology of
HIV & Hepatitis Therapy; June 8-10, 2016; Washington, DC
H. Sevinsky1, M. Chang2, J. Karkas2, D. Hawthorne1, P. Ravindran1, T. Eley1
1Bristol-Myers Squibb, Research and Development, Princeton NJ, USA; 2Bristol-Myers Squibb, Research and Development, Pennington NJ, USA
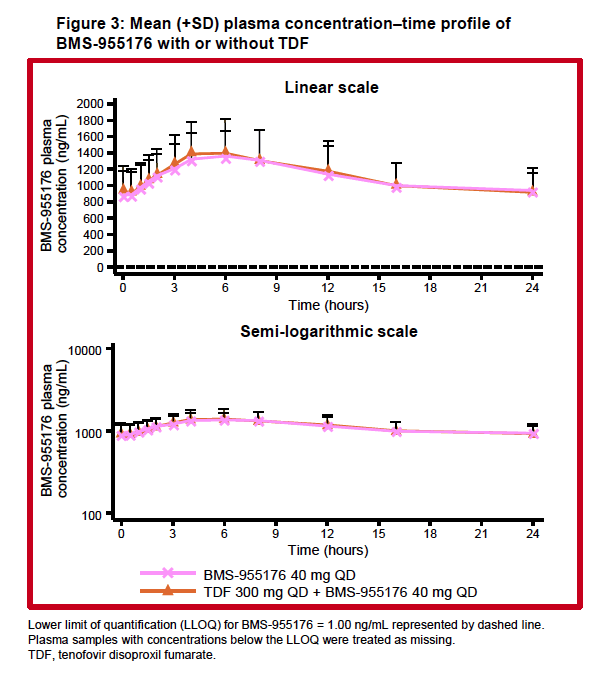
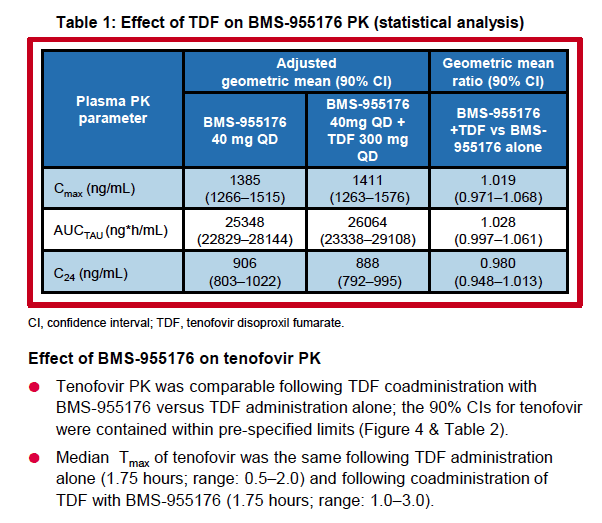
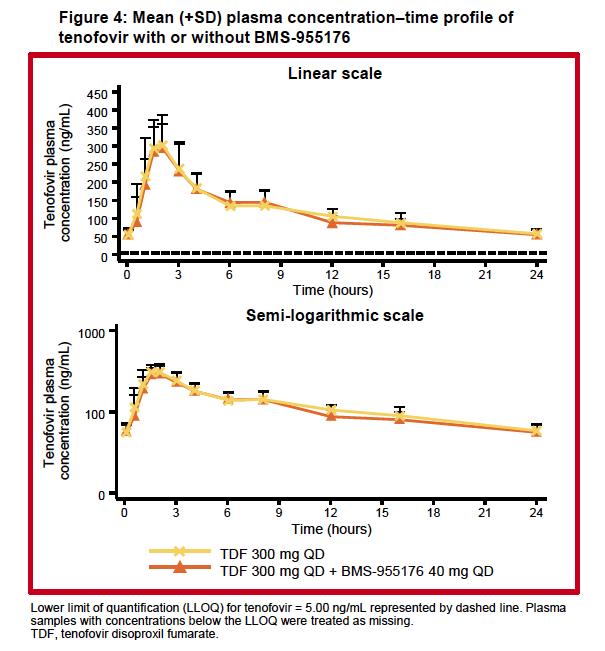
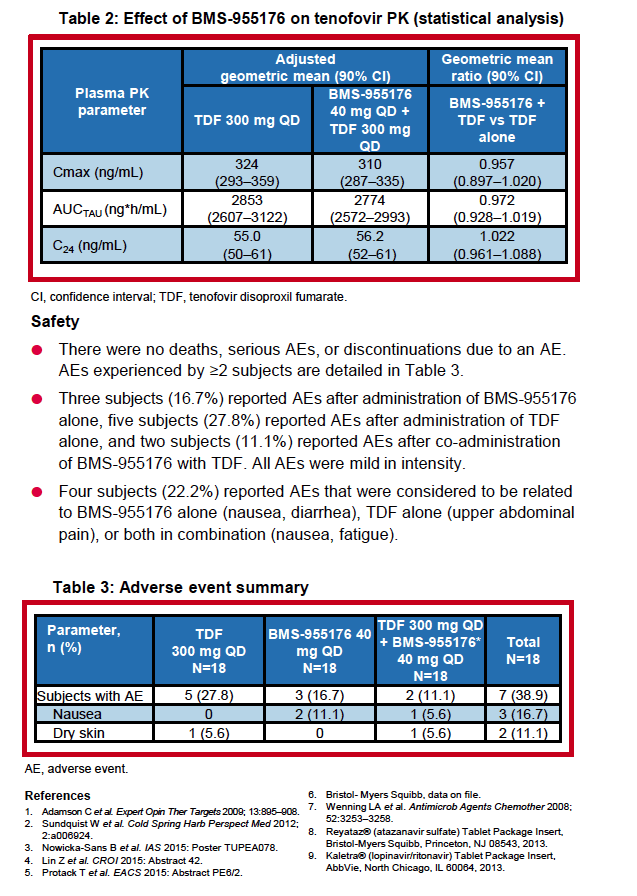
|
| |
|
 |
 |
|
|