 |
 |
 |
| |
AASLD 2017 Viral Hepatitis Debrief
|
| |
| |
Download the PDF here
Reported by Jules Levin
AASLD Annual Meeting 2017, Washington, DC, USA, October 20-24
Paul Y Kwo MD
Professor of Medicine
Director of Hepatology
Stanford University School of Medicine

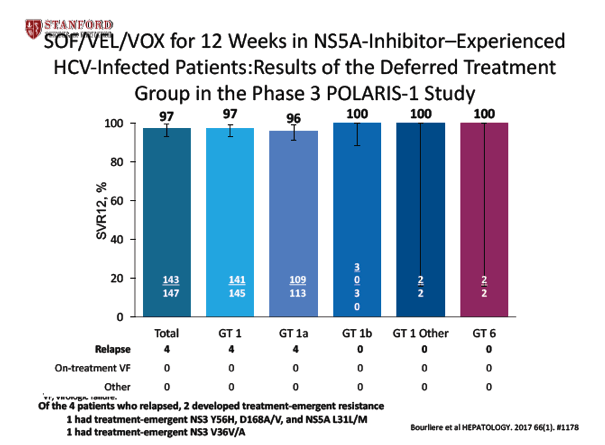
SOF/VEL/VOX for 12 Weeks in NS5A-Inhibitor-Experienced HCV-Infected Patients: Results of the Deferred Treatment Group in the Phase 3 POLARIS-1 Study - (10/23/17)
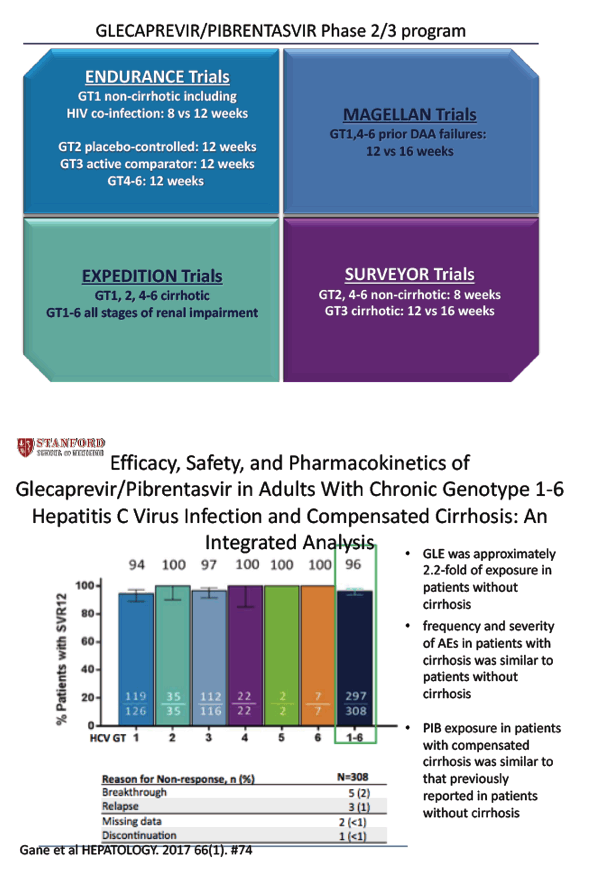
Efficacy, Safety, and Pharmacokinetics of Glecaprevir/Pibrentasvir in Adults With Chronic Genotype 1-6 Hepatitis C Virus Infection and Compensated Cirrhosis: An Integrated Analysis - (10/24/17)
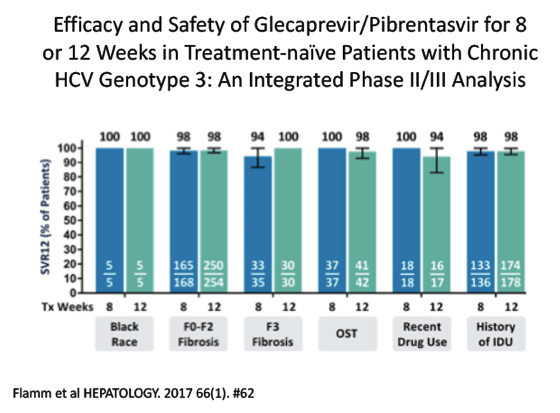
Efficacy and Safety of Glecaprevir/Pibrentasvir for 8 or 12 Weeks in Treatment-naïve Patients with Chronic HCV Genotype 3: An Integrated Phase 2/3 Analysis - (10/23/17)
A5327 Sofosbuvir-Containing Regimens Without Interferon for Treatment of Acute HCV in HIV-1-infected Individuals (SWIFT-C) - (10/24/17)
Safety and Efficacy of Treatment With Once-Daily Ledipasvir/Sofosbuvir (90/400 mg) for 12 Weeks in Genotype 1 HCV-Infected Patients With Severe Renal Impairment - (10/23/17)
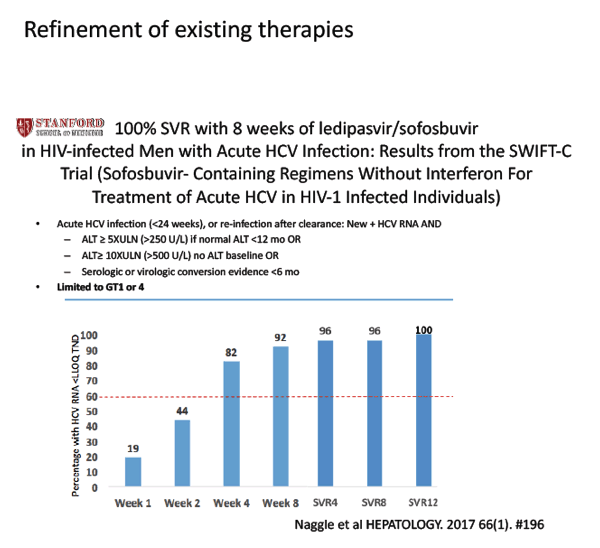
Sofosbuvir/Velpatasvir for 12 Weeks in Genotype 1-4 HCV-Infected Liver Transplant Recipients - (11/27/17)
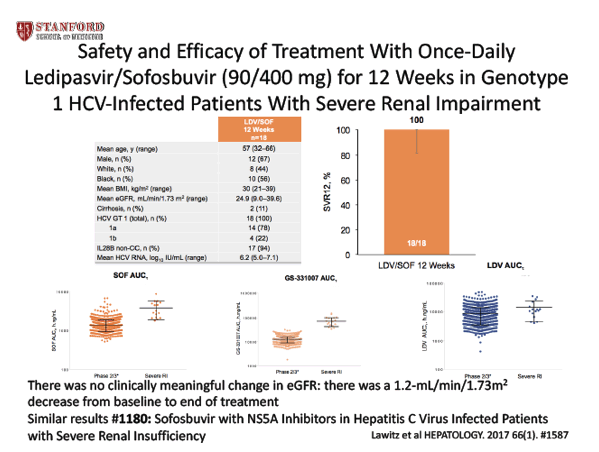
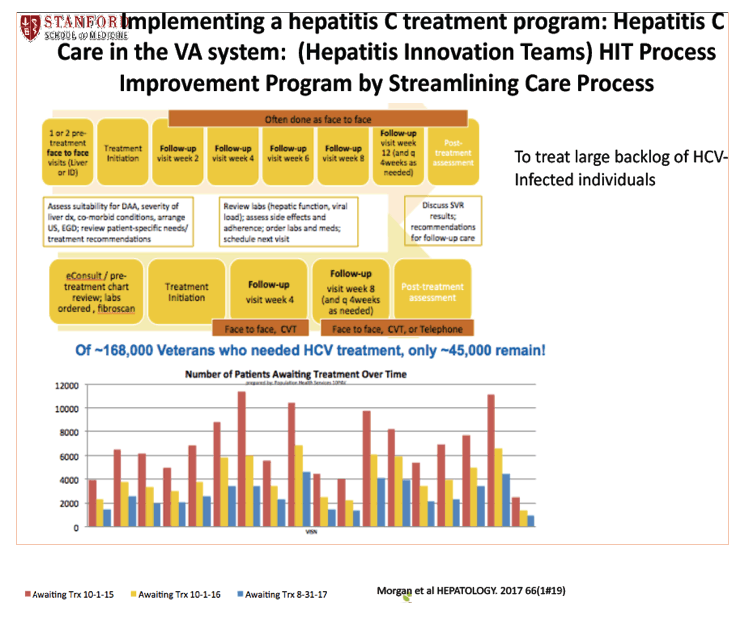
Effectiveness of Elbasvir/Grazoprevir in Patients With Chronic Hepatitis C and Chronic Kidney Disease:Results From the Veterans Affairs System - (11/27/17)
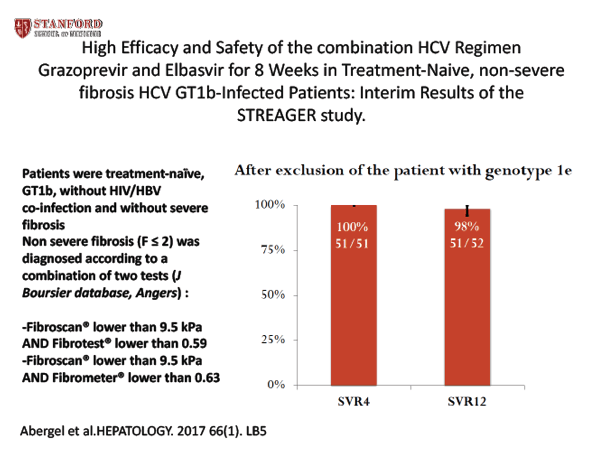
A Phrase 3, French Multicenter, Open-Label Study to Investigate the Efficacy of Elbasvir/Grazoprevir Fixed-Dose Combination for 8 Weeks in Treatment-Naïve HCV GT1b-Infected Patients with non-severe Fibrosis: STREAGER study - (10/24/17)
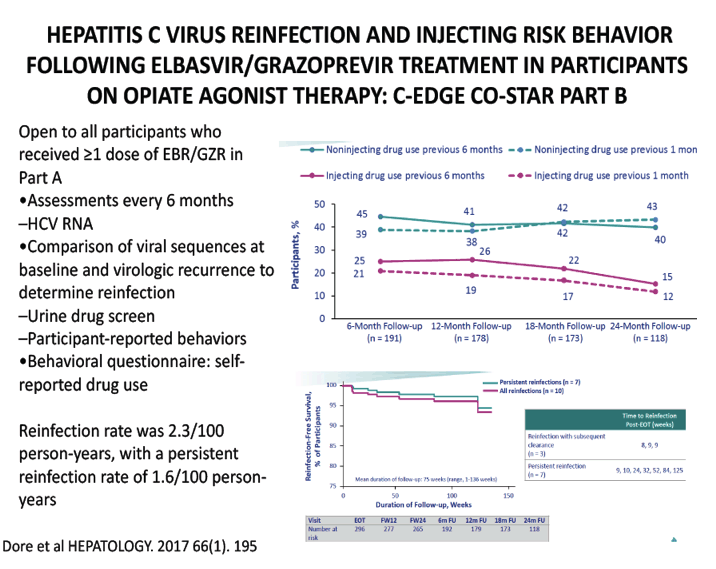
HEPATITIS C VIRUS REINFECTION AND INJECTING RISK BEHAVIOR FOLLOWING ELBASVIR/GRAZOPREVIR TREATMENT IN PARTICIPANTS ON OPIATE AGONIST THERAPY: C-EDGE CO-STAR PART B - (10/26/17)
INCIDENCE OF HEPATITIS C AMONG HIV-INFECTED MEN WHO HAVE SEX WITH MEN IN SAN DIEGO, 2000-2015 - (11/10/17)
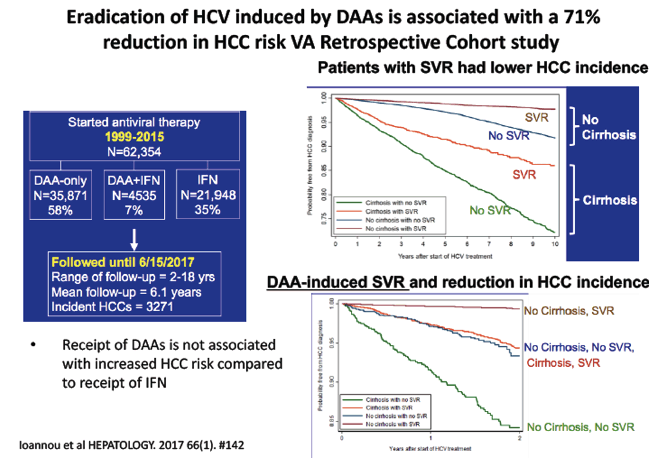
Eradication of HCV induced by DAAs is associated with a 71% reduction in HCC risk - (10/24/17)
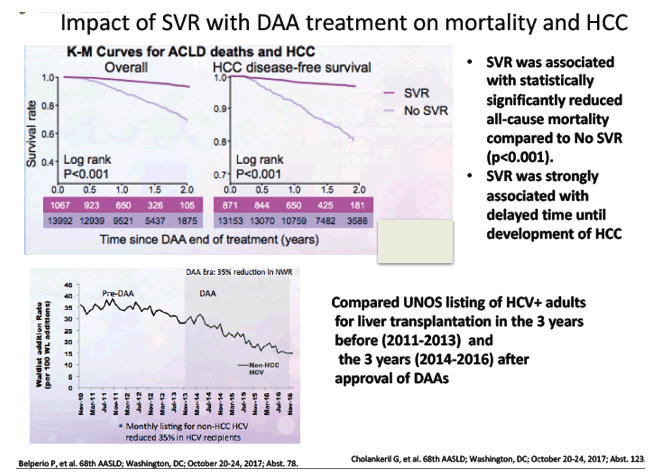
Impact of Sustained Virologic Response with Direct-Acting Antiviral Treatment on Mortality and Hepatocellular Carcinoma - significantly lower mortality, HCC 60% to 84% - (10/24/17)
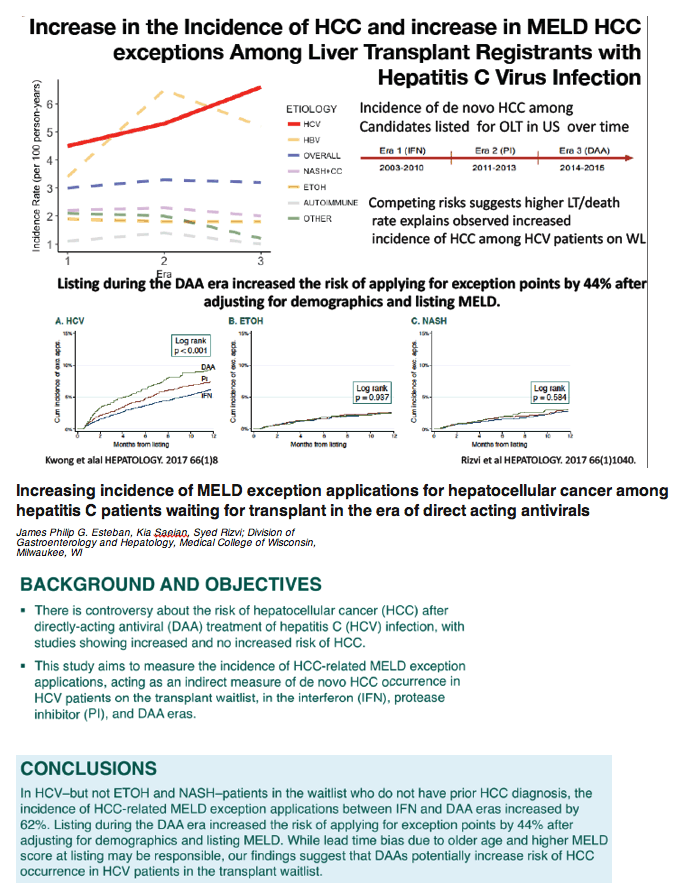
The Declining Burden of HCV on the Liver Transplant Waitlist associated with the DAA era - (11/13/17)
pdf of poster attached here
Download the PDF here
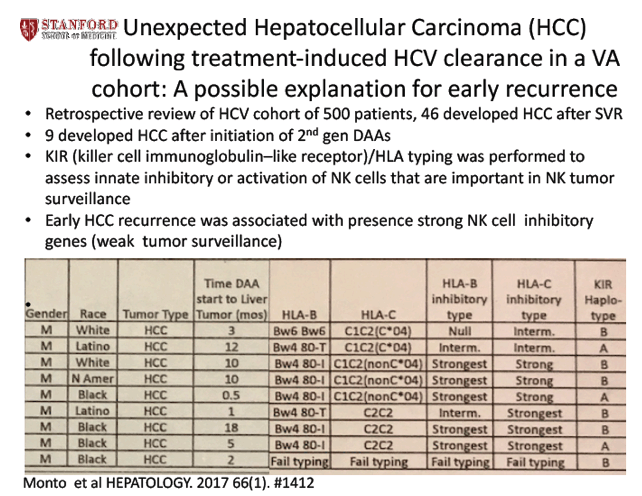
ABSTRACT BODY:
Introduction: During the longitudinal routine follow up of a long-term cohort of HCV patients, we observed an unexpectedly high incidence of HCC following treatment-induced viral clearance. Available data were retrospectively correlated with the development of HCC.
Methods: We analyzed immune response genotypes and clinical outcomes in a cohort of HCV seropositive subjects with chronic HCV. Subjects were typed for KIR, HLA, and for IL28B SNPs.
Results: 404 BASIC-HepC cohort patients with chronic HCV were followed; 159 IFNa-based treatment courses were given to 107 cohort patients, with 49 subjects (30.8%) eventually achieving SVR. 28 of these subjects had treatment that included a 1st generation direct antiviral (IFNα/Ribavirin/Telaprevir-Boceprevir); 8 (28.6%) achieved SVR. 169 treatment courses of 2nd generation direct antivirals were given to 164 subjects, with 153/164 subjects (93.2%) achieving SVR. A total of 92/404 subjects (22.8%) developed cirrhosis prior to any antiviral therapy, but cirrhosis developed later in an additional 28 of 74 subjects (37.8%) following failed IFNα-based treatment. A total of 46 subjects developed HCC, mostly in the presence of cirrhosis. HCC was diagnosed in 20/404 subjects (5.9%) prior to therapy, in 4/74 subjects (5.4%) after failed IFNα treatment, in 4/49 subjects (8.2%), after IFNa-based SVR. An additional 3/28 subjects (10.7%) developed HCC after treatment with 1st generation antivirals (2 after SVR, 1 in a relapser during the aviremic window), and 11/164 subjects (6.7%) developed HCC after the initiation of 2nd generation antiviral treatment, (8 after SVR and 3 in relapsers during the aviremic window). The median time to HCC was 54.3 (48-67) months after IFNα-based treatment, 13.3 (2-48) months after 1st generation antivirals, and 5.5 (0.5-18) months after exposure to 2nd generation antivirals. Cirrhosis was present in only 7/11 subjects with HCC following 2nd generation antiviral treatment. All 11 subjects with HCC after 2nd generation antivirals had longstanding HCV, with a mean infection duration of 44 (36-50) years, and many were ethnic minorities; 5 were Black, 4 were Latino/Native American. All 11 subjects had strong NK cell inhibitory KIR/HLA types such as KIR2DL1/HLA-C2 (often with KIR3DL1/HLA-Bw4), suggesting diminished tumor immunosurveillance.
Conclusions: Compared to earlier therapies, there was an unexpected temporal association between 2nd generation antiviral therapy and the rapid development of HCC, even in the absence of cirrhosis. HCC after 2nd generation treatments may be associated with a long duration of infection, minority ethnicity, and with weak NK cell tumor surveillance,
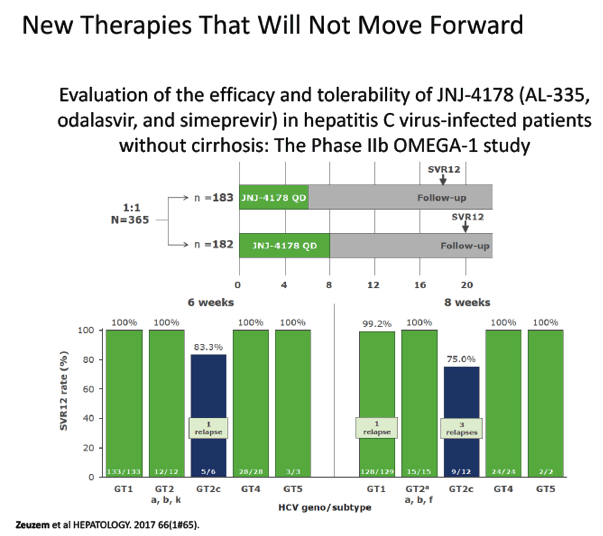
Evaluation of the efficacy and tolerability of JNJ-4178 (AL-335, odalasvir, and simeprevir) in hepatitis C virus-infected patients without cirrhosis: The Phase IIb OMEGA-1 study - (10/23/17)
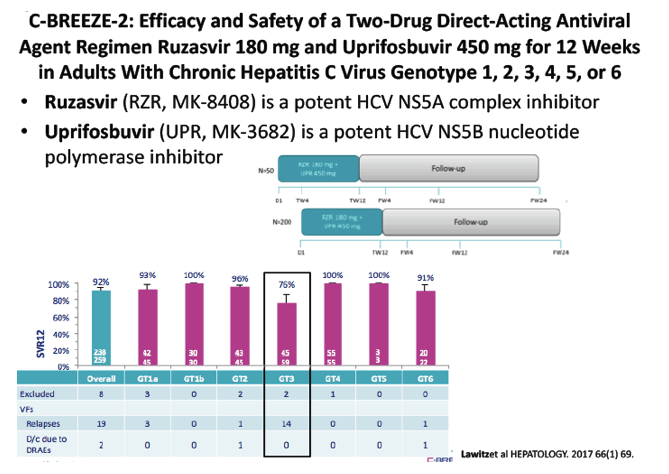
C-BREEZE-2: Efficacy and Safety of a Two-Drug Direct-Acting Antiviral Agent Regimen Ruzasvir 180 mg and Uprifosbuvir 450 mg for 12 Weeks in Adults With Chronic Hepatitis C Virus Genotype 1, 2, 3, 4, 5, or 6- (10/23/17)
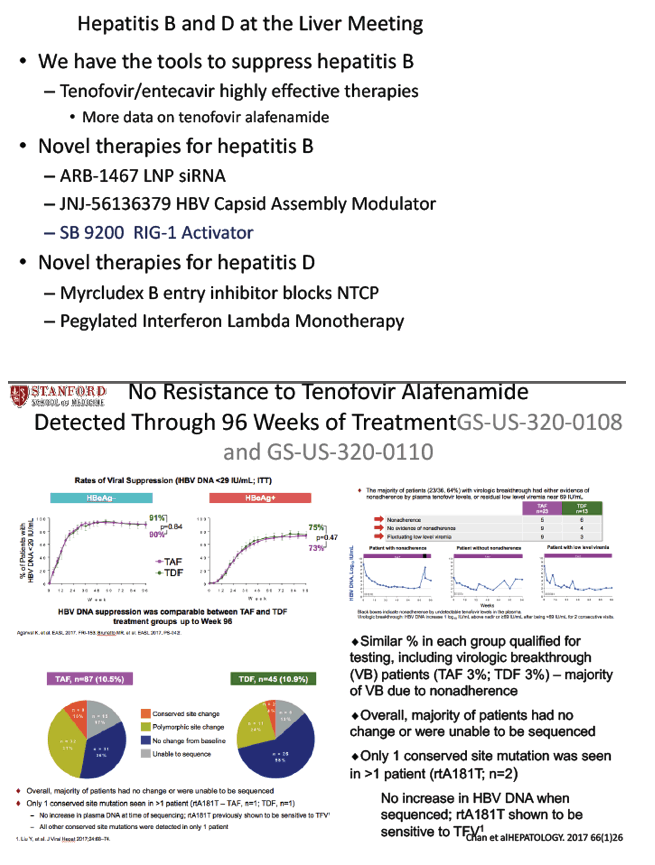
No Resistance to Tenofovir Alafenamide Detected Through 96 Weeks of Treatment in Patients with Chronic Hepatitis B - (10/26/17)
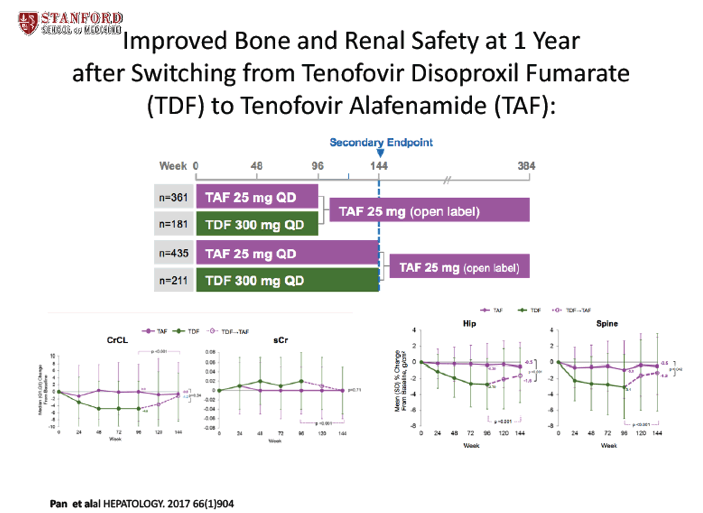
Improved Bone and Renal Safety at 1 Year After Switching From Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide: Results From 2 Phase 3 Studies in HBeAg-Positive and HBeAg-Negative Patients With Chronic Hepatitis B - (11/08/17)
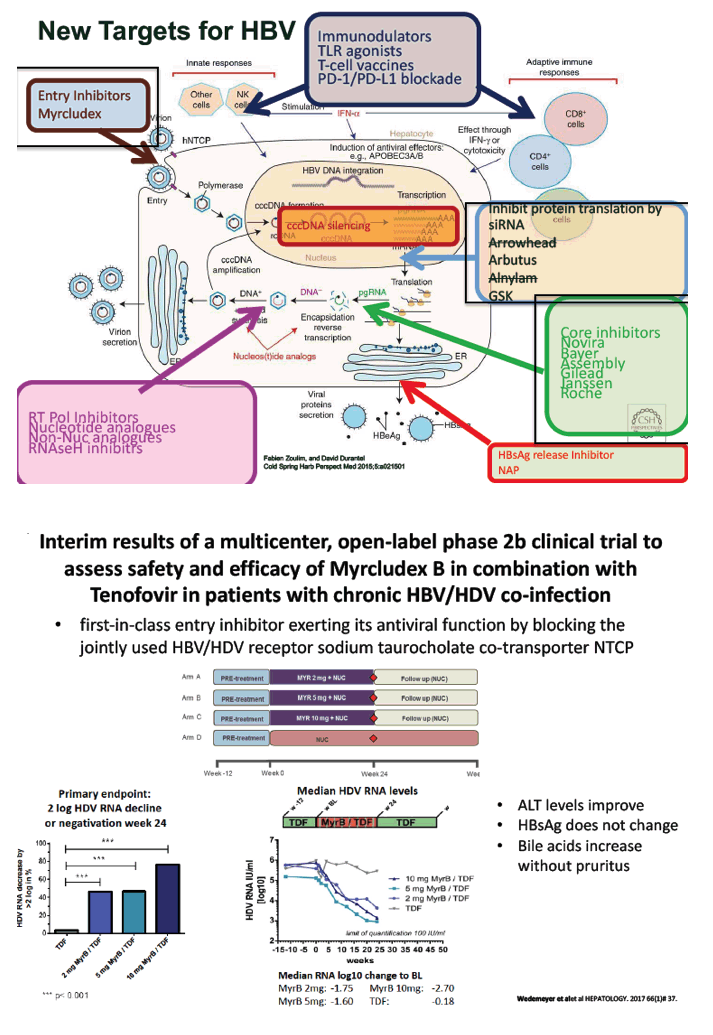
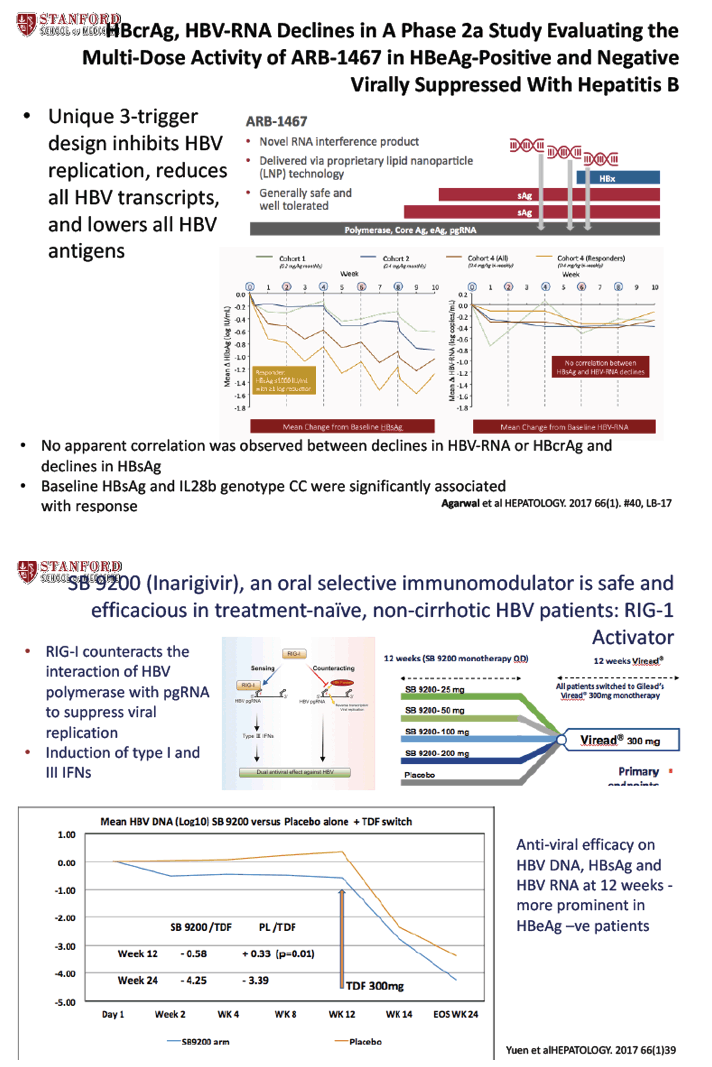
Novel anti-viral activity of SB 9200, a RIGI agonist; results from Cohort 1 of the ACHIEVE trial - (11/08/17)
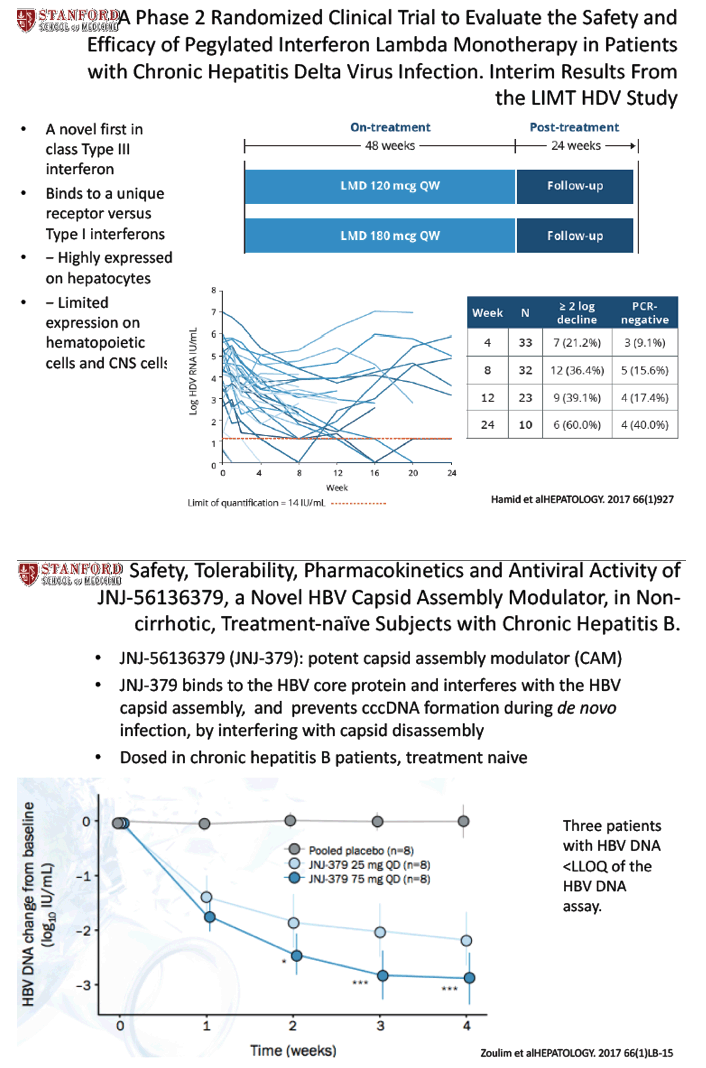
Safety, Tolerability, Pharmacokinetics, and Antiviral Activity of JNJ-56136379, a Novel HBV Capsid Assembly Modulator, in Non-cirrhotic, Treatment-naïve Patients with Chronic Hepatitis B. - (11/01/17)
|
| |
|
 |
 |
|
|