 |
 |
 |
| |
Randomized Controlled Trial of Cash Incentives or Peer Mentors to Improve HCV Linkage and Treatment Among HIV/HCV Coinfected Persons Who Inject Drugs: The CHAMPS Study
|
| |
| |
from Jules: support service like these outlined in this study are important to treating IDUs and drug users, and to help education to prevent reinfection which is a key reason insurers do not want to pay for treatment. So a full program of support services for IDUs and drug users and marginalized patient populations can be useful in convincing government officials and insurers to treat. The State, City and Federal government should be funding these support services programs, and these programs should be part of any HCV Elimination Programs in order for them to be successful. These types of services were part of the original NYC screening & linkage to care program I designed 6 years ago that ultimately was called Check Hep C program. My original concept was not exactly implemented but over time all have learned a lot. Still overall screening & linkage to care remains a big challenge, without full funded screening & linkage programs as part of large HCV Elimination Programs we will always be grasping to handle the HCV problem, HCV elimination which was an attainable vision when we had the new DAAs and 95%+ cure rates will continue to elude us without compressive & fully funded HCV Elimination Programs. The federal, city and state governments continue in their refusal to implement full screening & linkage programs and HCV Elimination Programs.
Reported by Jules Levin
AASLD: The Liver Meeting® 2017, October 20-24, 2017, Washington, DC
Ward K1,Falade-Nwulia O1, Moon J1, Sutcliffe C2, Brinkley S1, Haselhuhn T1, Thomas D1, Katz S1, Herne K1, Arteaga L1, Mehta S2, Sulkowski M1
1Department of Medicine, Johns Hopkins School of Medicine and 2Department of Epidemiology Johns Hopkins Bloomberg School of Public Health, Baltimore MD, USA

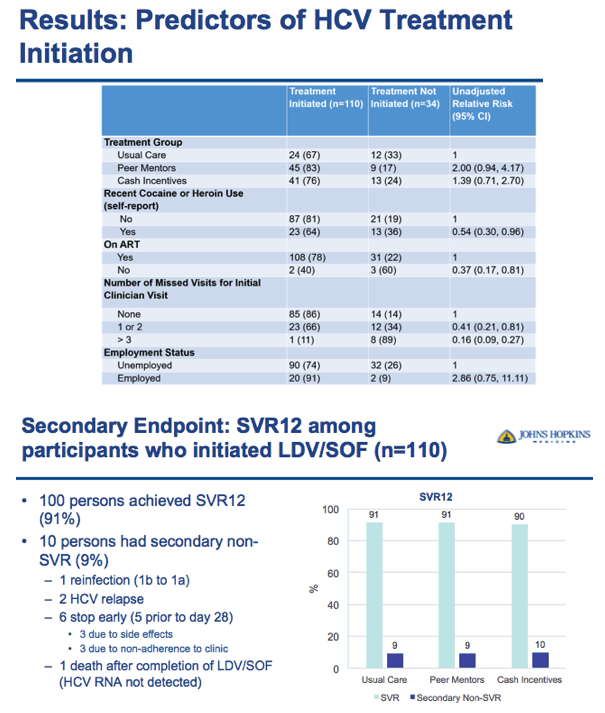
⋅But it was also clear to us that barriers to treatment remain because despite access to treatment and on-site HCV clinicians, we identified ~560 coinfected patients that were engaged in HIV care but were not engaged in HCV care
⋅These patients remained at risk for progressive liver disease and HCV-related mortality

⋅To address this need we developed The CHAMPS Study with the hypothesis that Novel interventions, like peer mentors and contingent financial incentives, will increase HCV treatment initiation and cure in HIV/HCV coinfected persons who use drugs. We will compare these interventions to those receiving usual clinical care
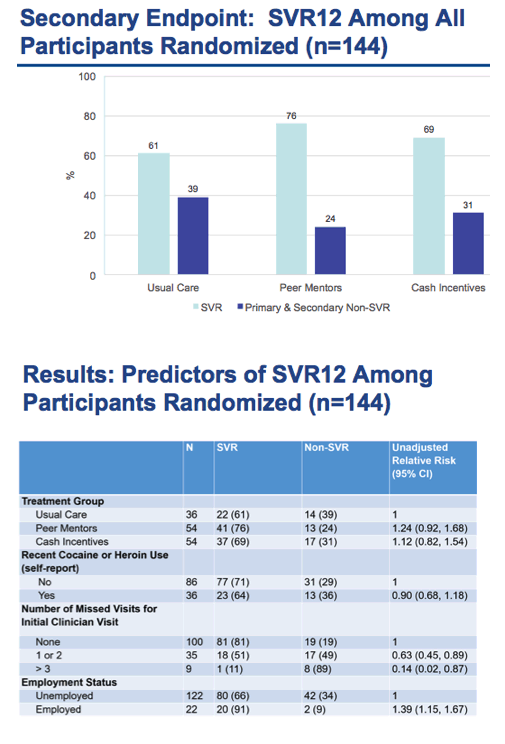
⋅If we turn to the HCV care continuum, we are focusing our efforts first on the linkage to care step then with the interventions like peer mentors, we are also addressing other steps of the continuum like treatment, cure and even post-cure care
⋅Today's presentation will be a discussion of preliminary findings presented at EASL 2017 with the final data being presented at AASLD Liver Meeting next month.
⋅
⋅(don't say) Adherence to treatment schedule is also a primary hypothesis to be tested based on: 1) Proportion of visits attended since clinic attendance allows for the delivery of care and medication (number of visits attended/expected); 2) Proportion of treatment days received (maximum 84 days); 3) Proportion of tablets received (maximum, 84 tablets).
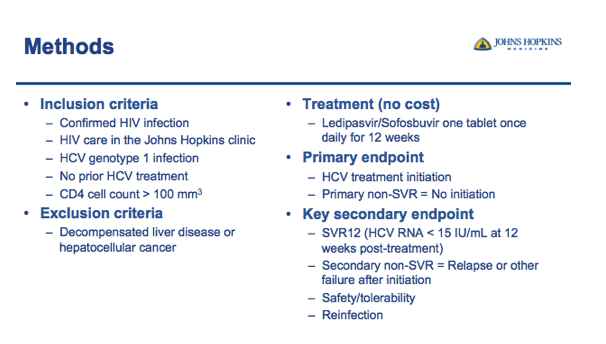
from Jules: I have been suggesting "pay for cure" for years, by incentivizing treatment by paying patients to get tested, for each followup visit, for each stage of evaluation treatment, and if ultimately competing treatment & being cured. And I would suggest considering increasing to even higher than the $220 paid here.
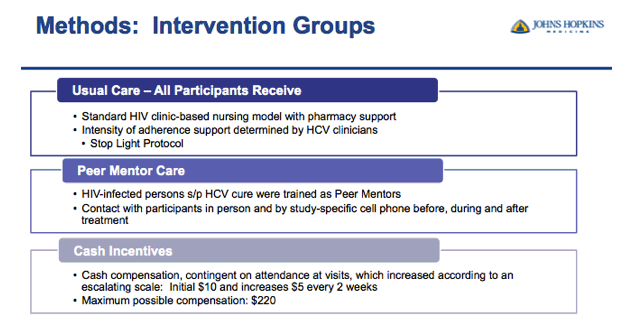
⋅First I will go into more detail about the 3 treatment groups
⋅All participants enrolled in the study received usual care
⋅But when I say "usual care" it is probably a little different from what you are imagining because the Johns Hopkins HIV Clinic initiated a clinic-based nursing model to help patients through HCV treatment called the stop light protocol.
⋅Patients were assigned a level of adherence support by their HCV provider, either Green, Yellow or Red
⋅Green level patients receive minimal intervention and are permitted to initiate HCV treatment on their own
⋅Yellow and Red level patients receive moderate to significant intensity support including a mandatory nursing initiation visit to start LDV/SOF; followed by frequent, up to weekly, visits and or phone calls with their nurse during the course of therapy
⋅The CHAMPS Study was enrolling patients in the yellow and red categories so all patients were receiving adherence support via the clinic based nursing model
⋅
⋅Participants in the Peer Mentor group will receive the usual care model and also be assigned a peer mentor
⋅Peer mentors are trained HIV positive patients from the clinic that have successfully completed HCV treatment and achieved SVR
⋅They were recruited from active support groups in the clinic so all peers had experience as advocates in the community.
⋅In order to remain in contact with their patients they were given a study cell phone and logs to track patient progress
⋅The main goal of the peer mentors was to be a support system for their patients before, during and after treatment with in person and phone communication
⋅
⋅Finally, Participants in the cash incentives group, in addition to receiving the usual care model, also received cash incentives on an escalating scale.
⋅First patients were compensated $10 for starting treatment then the incentive would increase by $5 as they attended clinic visits every two weeks during treatment.
⋅We are incentivizing attendance to the clinic visit and if the patient attends all visits without missing they can earn up to $220
⋅If they missed visits without notifying the study coordinator to reschedule, their escalating scale restarts at $10
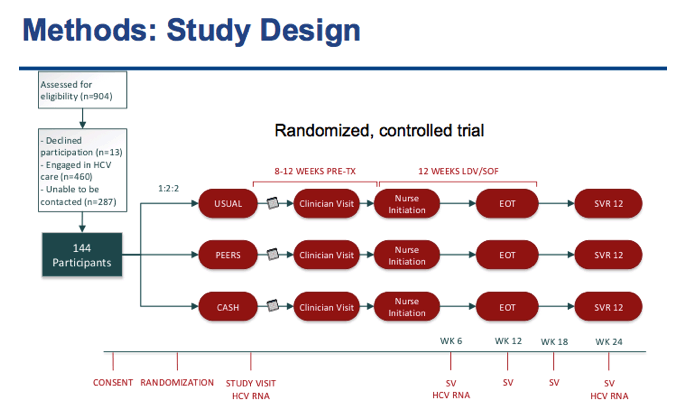
⋅After recruitment, We randomized 144 Participants who were HCV GT 1, treatment naïve with no evidence of decompensated liver disease
⋅All participants were co-infected with HIV and had a CD4 count above 100
⋅They were patients engaged in the JH HIV Clinic with a provider they could see for follow-up
⋅But they were not engaged in HCV care at all or had fallen out of care and had not be seen for 8 months or more.
⋅After consent, the participants were randomized 1:2:2 into 1 of the 3 treatment groups
⋅Once Randomized participants have their first study visit where they would schedule an appointment with a clinician. The study visits are kept separate from clinical evaluations in order to mimic usual care as closely as possible.
⋅The clinician will determine if the patient needs a HIV regimen change or any other clinical tests like an Ultrasound.
⋅After the study visit, All patients have 8 weeks to see a provider and follow-up with a nurse for their nursing initiation visit
⋅Patients that need to change their HIV regimen have 12 weeks
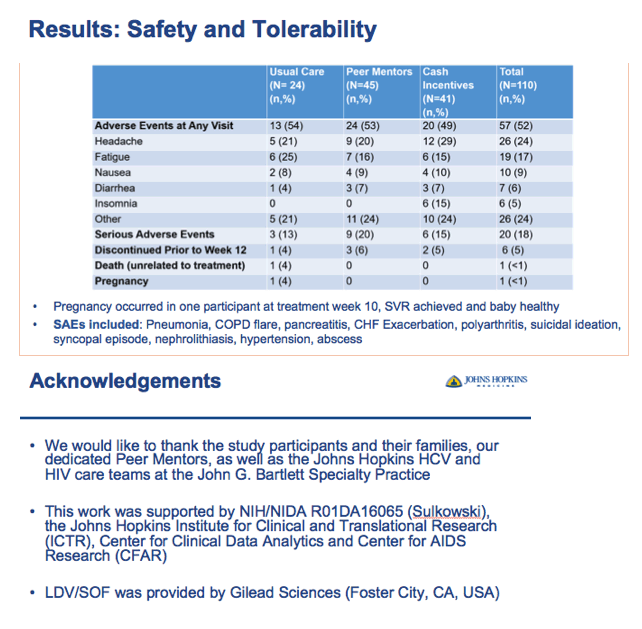
⋅Nurses will start the patient on LDV/SOF at no cost per the stop light protocol and follow up throughout and after treatment as needed
⋅Study visits occur at weeks 6 and 12 during treatment and at post-treatment weeks 6 and 12
⋅Cash Incentive group participants will have additional study visits at two week intervals during treatment.
|
| |
|
 |
 |
|
|