 |
 |
 |
| |
A Randomized, Double-Blinded, Placebo-Controlled Trial of the Dipeptidyl Peptidase-4 (DPP-4) Inhibitor Sitagliptin for Reducing Inflammation and Immune Activation in Treated and Suppressed HIV Infection ACTG 5346
|
| |
| |
Reported by Jules Levin
19th International Workshop on Co-morbidities and Adverse Drug Reactions in HIV, Milan, Italy Oct 23-25 2017
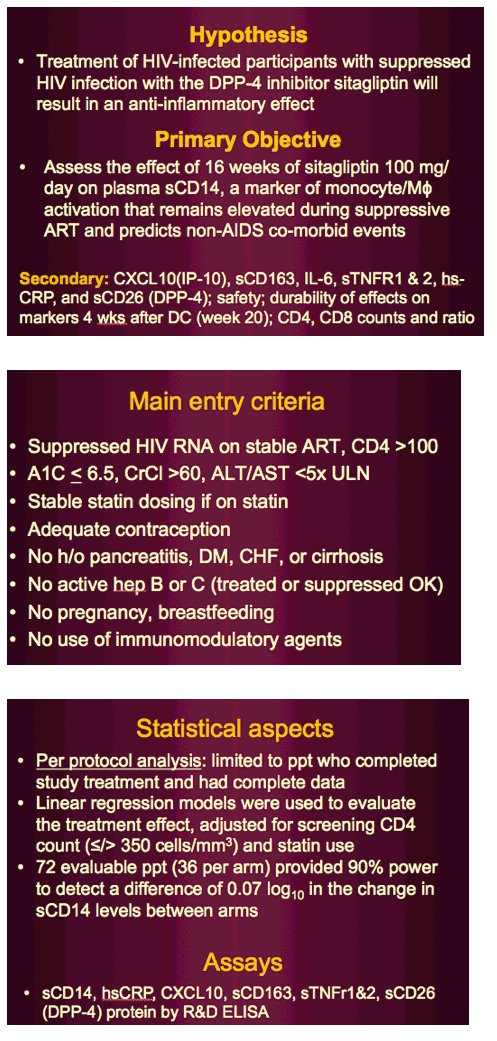
MP Dube, ES Chan, JE Lake, B Williams, J Winslow, A Landay, RW Coombs, HJ Ribaudo, KE Yarasheski
Download the PDF here

pdf attached
Sitagliptin Reduces Inflammation and Chronic Immune Cell Activation in HIV+ Adults With Impaired Glucose Tolerance JCEM 2015
People living with HIV infection experience a 2-fold greater risk of vascular disease, myocardial infarction, or stroke, as well as a 2- to 4-fold greater incidence of elevated fasting glucose or hyperinsulinemia than the general population (1–3). The underlying mechanisms remain unclear. These non-AIDS comorbidities persist despite combination antiretroviral therapy (cART) and HIV suppression. They have been associated with chronic low-grade systemic inflammation, residual immune cell activation, and monocyte-macrophage migration into adipose depots (4, 5). Therapies targeted at reducing chronic immune cell activation and inflammation have been tested in cART-treated HIV-infected (HIV+) adults with suppressed viremia (6). However, no safe and effective treatment for reducing inflammation and immune cell activation in HIV+ adults exists.
Sitagliptin is a dipeptidyl peptidase-4 (DPP4) inhibitor (DPP4i) that represents a growing class of antidiabetic drugs that inhibit DPP4 enzyme activity and an exopeptidase that cleaves two N-terminal amino acids from the incretin hormones glucagon-like peptide (GLP)-1 and glucose-dependent insulinotropic polypeptide (GIP), which restricts their actions (7, 8). Sitagliptin inhibits circulating soluble DPP4 (sDPP4) enzyme activity and prolongs the circulating half-life of GLP-1 and GIP, indirectly enhancing insulin secretion and action (9). GLP-1 and GIP activate ubiquitously expressed G protein-coupled receptors (9) that exert several metabolic actions (9–14).
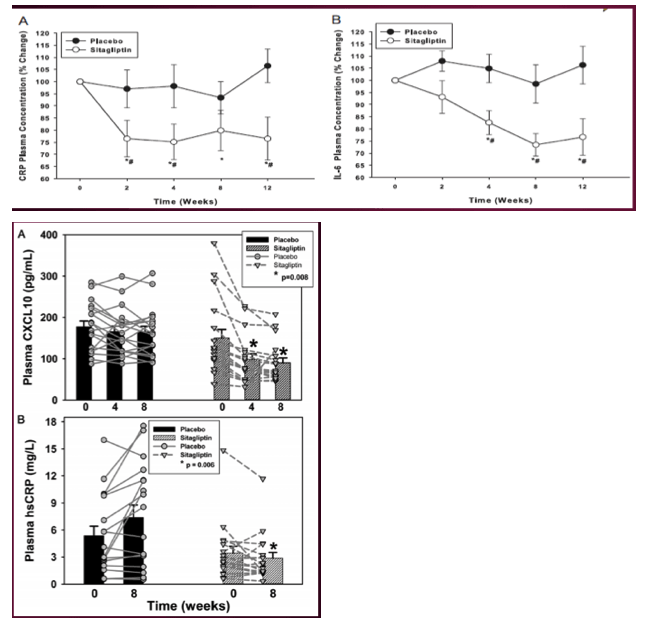
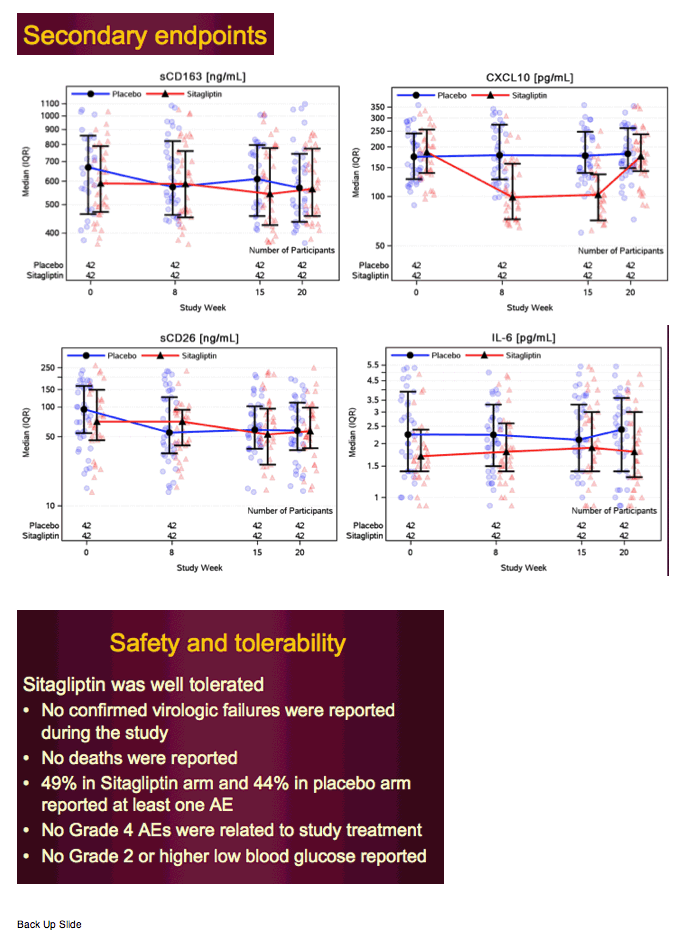
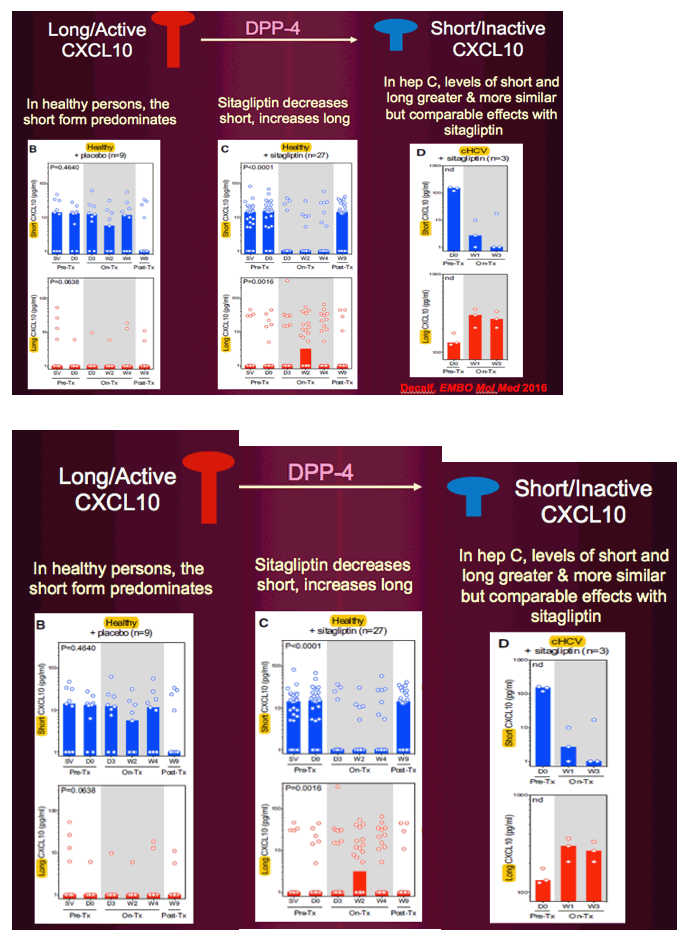
The findings suggest that 8 weeks of sitagliptin had beneficial anti-inflammatory, immune regulatory, hematopoietic progenitor cell-mobilizing, and glucose-lowering effects in cART-treated virally suppressed HIV+ adults with IGT. In cART-treated HIV+ adults, plasma hsCRP is a marker for persistent immune cell activation and is associated with greater vascular endothelial activation markers, rates of thrombosis, and carotid intima media thickness—CVD risk factors (25–27). In this study, sitagliptin reduced plasma hsCRP concentration, whereas hsCRP increased in placebo-treated participants. Sitagliptin reduced the concentration of plasma CXCL10, a chemokine secreted from monocytes and endothelial cells in response to interferon-γ, HIV-related proteins (Tat, Nef, gp120), and lipopolysaccharide (LPS) binding to toll-like receptor-4 present on immune cells (28).
Context:
HIV infection is associated with a greater risk for fasting hyperinsulinemia, impaired glucose tolerance, and higher incidence rates for vascular disease, myocardial infarction, or stroke despite effective combination antiretroviral therapy (cART). The underlying mechanism(s) may involve chronic low-grade systemic inflammation and immune cell activation. Dipeptidyl peptidase-4 inhibitors (sitagliptin) improve glucose tolerance and may possess immunomodulatory effects because leukocyte CD26 cell surface receptors express dipeptidyl peptidase-4 activity.
Objective:
Sitagliptin will reduce inflammatory and immune cell activation markers known to be elevated in cART-treated HIV-infected (HIV+) adults with impaired glucose tolerance.
Design:
This was designed as a prospective, randomized, placebo-controlled, double-blind trial of sitagliptin in HIV+ adults.
Setting:
The setting was an academic medical center.
Patients:
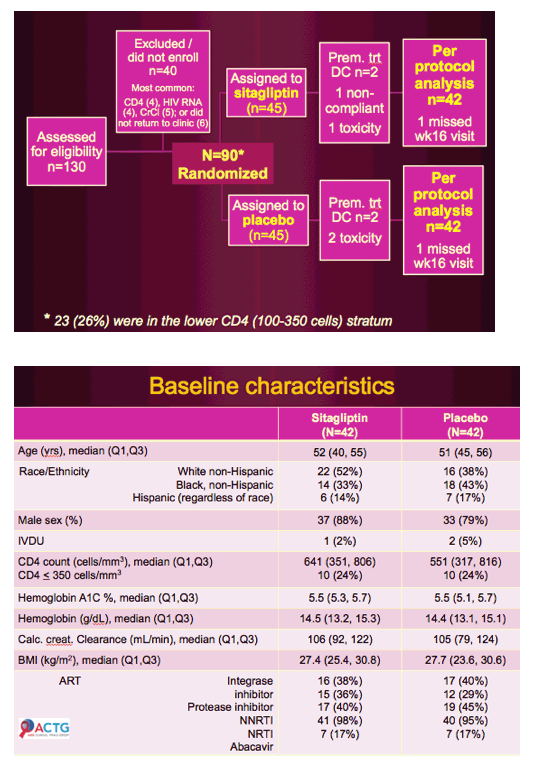
Patients were cART-treated HIV+ men and women (n = 36) with stable HIV disease and impaired glucose tolerance.
Interventions:
Interventions included sitagliptin 100 mg/d or placebo for 8 weeks.
Main Outcome Measures:
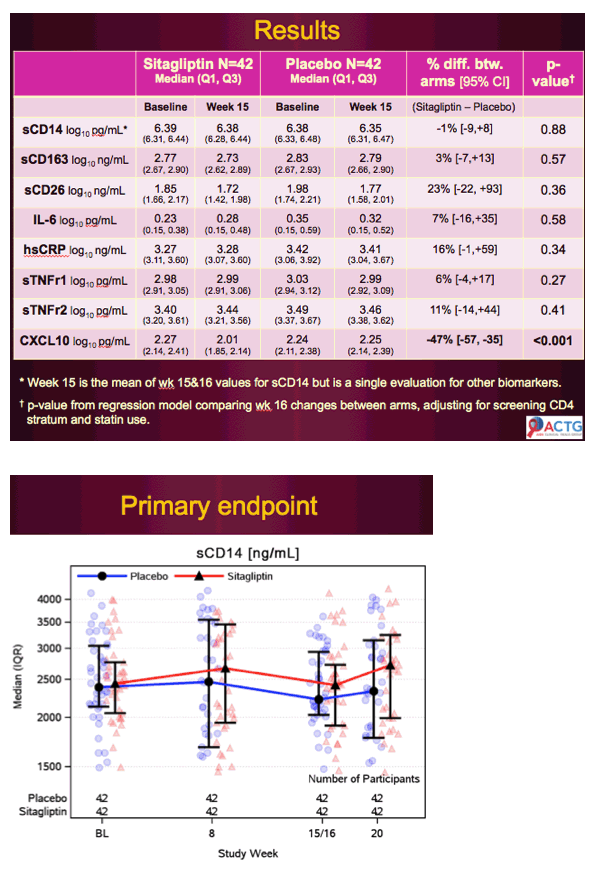
At baseline and week 8, plasma high-sensitivity C-reactive protein and C-X-C motif chemokine 10 concentrations (ELISA), oral glucose tolerance, and abdominal sc adipose mRNA expression for M1 macrophage markers (monocyte chemotactic protein-1, EGF-like module-containing, mucin-like hormone receptor 1).
Results:
Sitagliptin reduced glucose area under the curve (P = .002) and improved oral glucose insulin sensitivity index (P = .04) more than placebo. Sitagliptin reduced plasma high-sensitivity C-reactive protein and C-X-C motif chemokine 10 levels more than placebo(P < .009). Adipose tissue monocyte chemotactic protein-1 mRNA abundance declined significantly more (P = .01), and adipose EGF-like module-containing, mucin-like hormone receptor 1 mRNA expression tended to decline more (P = .19) in sitagliptin than placebo.
Conclusion:
Sitagliptin had beneficial systemic and adipose anti-inflammatory effects in cART-treated HIV+ adults with impaired glucose tolerance. Large-scale, long-term studies should determine whether sitagliptin reduces cardiovascular risk and events in HIV+ adults.

|
| |
|
 |
 |
|
|