 |
 |
 |
| |
Liver Cancer Cases Rise After SVR With All-DAA Therapy in Spanish HCV/HIV Cohort...... "our study has not found evidence for an increased HCC incidence with DAA use"
|
| |
| |
"....the proportion of HCC cases diagnosed in HIV/HCV coinfected patients with previous SVR has significantly increased parallel to the arrival of DAA IFN-free strategies....this finding may be, at least partially, explained by the fact that DAA have allowed treating patients at advanced stages of liver disease in which protective effect of SVR on the risk of HCC could be less marked"
....from Jules: delaying HCV treatment until patients have more advanced HCV disease allows for undetected or subclinical HCC or disease progression moving towards HCC so despite SVR liver disease may have progress.
Conference on Retroviruses and Opportunistic Infections (CROI), February 13-16, 2017, Seattle
WEBCAST:http://www.croiwebcasts.org/console/player/33569?mediaType=slideVideo&
Mark Mascolini
Among HCV/HIV-infected people with hepatocellular carcinoma (HCC), the proportion diagnosed after sustained virologic response (SVR) in the current direct-acting antiviral (DAA) era exceeded proportions diagnosed after SVR in three previous HCV treatment eras. Researchers who conducted this study in 319 HCV/HIV-infected people in Spain speculate that the jump in post-SVR HCC cases with DAAs could reflect the growing proportion of people with advanced liver disease being treated with DAAs.
Achieving SVR usually leads to lower risk of liver complications, including HCC, in people coinfected with HCV and HIV. But coinfected people with cirrhosis still run a risk of HCC after SVR. To track rates of post-SVR HCC across HCV treatment eras, a Spanish team analyzed data from the GEHEP-002 cohort of HIV-positive HCC patients at 32 centers in Spain [2].
This analysis focused on HCV/HIV patients diagnosed with HCC and divided into four treatment eras: Period 1 (before 2002) non-pegylated interferon (IFN); Period 2 (2002-2011) pegylated IFN plus ribavirin; Period 3 (2012-October 2014) DAAs combined with IFN; and Period 4 (October 2014-2016) DAA regimens without IFN. Median age stood at 49 (interquartile range 46 to 52), and 287 people (90%) were men. While 27% had a history of drinking more than 50 g of alcohol daily, 14% tested positive for hepatitis B surface antigen.
Of the 319 people with HCC, numbers and proportions diagnosed with HCC after SVR were 1 of 6 (16.7%) in Period 1, 16 of 161 (9.9%) in Period 2, 10 of 111 in Period 3 (9%), and 13 of 41 (31.7%) in the DAA-only Period 4. The difference in proportion of post-SVR HCC cases between Period 4 and Periods 1-3 was statistically significant (P < 0.001). The researchers could not calculate post-SVR HCC incidence.
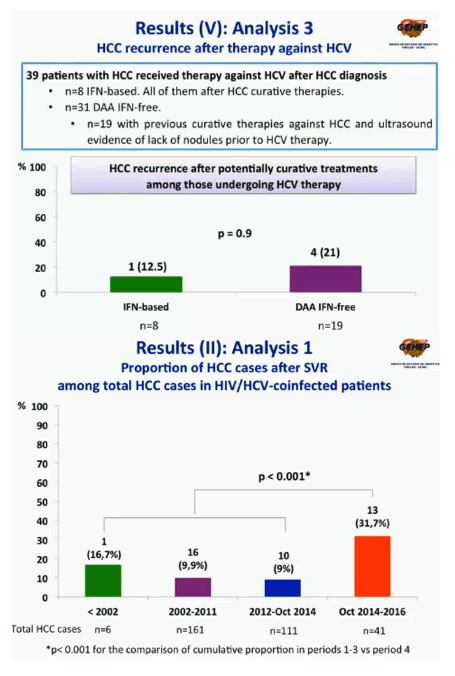
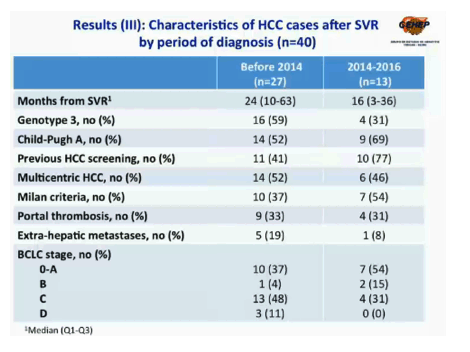
Comparing the 27 post-SVR HCC cases in Periods 1-3 to the 13 cases in Period 4, the researchers found that the Period 4 group had a shorter time from SVR to HCC (median 16 versus 24 months), a lower proportion with HCV genotype 3 (31% versus 59%), a higher proportion with Child-Pugh class A, the best-prognosis class (69% versus 52%), a higher proportion with previous HCC screening (77% vs 41%), and a lower proportion with extrahepatic metastases (8% versus 19%).
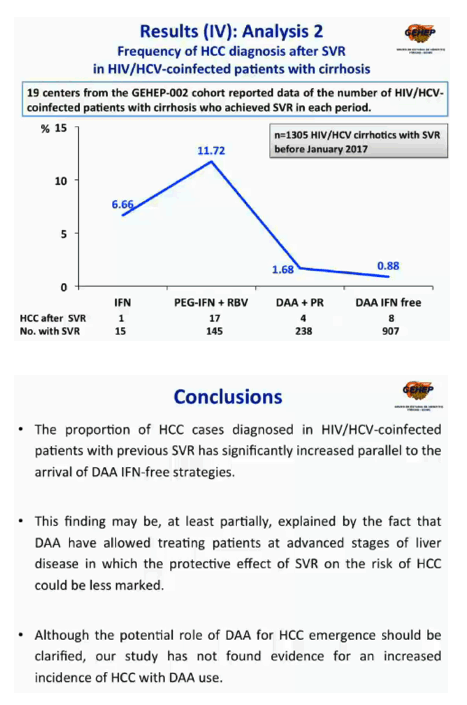
Before January 2017 the cohort included 1305 HCV/HIV patients with cirrhosis who achieved SVR: 15 in Period 1, 145 in Period 2, 238 in Period 3, and 907 in Period 4. Numbers and proportions diagnosed with HCC after SVR were 1 (6.7%) in Period 1, 17 (11.7%) in Period 2, 4 (1.7%) in Period 3, and 8 (0.9%) in the DAA-only Period 4. Eight people who received an IFN-based regimen and 19 who received a DAA-only regimen had potentially curative therapy for HCC. One of 8 people (12.5%) in the IFN group had HCC recurrence, compared with 4 of 19 (21%) in the DAA-only group, a nonsignificant difference (P = 0.9).
The GEHEP team suggests that the higher proportion of HCC diagnoses after SVR in the DAA-only era may partly reflect treatment of more people with advanced liver disease with DAAs. A much higher number of HCV/HIV patients with cirrhosis received anti-HCV therapy in the DAA-only period than in the three earlier periods combined (907 vs 398). And that could diminish the protective effect of SVR on HCC risk. The researchers recommend that "the potential role of DAAs for HCC emergence should be clarified."
References
1. Merchante N, Revollo B, Rodriguez-Arrondo F, et al. Hepatocellular carcinoma after SVR with IFN-free regimens in HIV/HCV coinfection. Conference on Retroviruses and Opportunistic Infections (CROI), February 13-16, 2017, Seattle. Abstract 139. http://www.croiconference.org/sessions/hepatocellular-carcinoma-after-svr-ifn-free-regimens-hivhcv-coinfection
2. ClinicalTrials.gov. Hepatocellular carcinoma in HIV-infected patients (GEHEP-002). ClinicalTrials.gov identifier NCT02785835. https://clinicaltrials.gov/ct2/show/NCT02785835
|
| |
|
 |
 |
|
|