 |
 |
 |
| |
Dolutegravir as Maintenance Monotherapy for
HIV-1: a Randomized Clinical Trial
|
| |
| |
Reported by Jules Levin
CROI 2017 Feb 14-16 Seattle, WA
I.E.A.Wijting1, C. Rokx1, C.A.B. Boucher2, T.E.M.S. de Vries-Sluijs1, C.A.M. Schurink1, E.R. Andrinopoulou3, E.C.M. van Gorp1,
W.F.W. Bierman4 and B.J.A. Rijnders1 on behalf of the DOMONO study-group
1 Department of Internal Medicine and Infectious Diseases, Erasmus MC, Rotterdam, The Netherlands, 2 Department of Virosciences, Erasmus MC, Rotterdam, The Netherlands,
3 Department of Biostatistics, Erasmus MC, Rotterdam, The Netherlands, 4 Department of Internal Medicine, Infectious Diseases Service, University of Groningen, University Medical Center Groningen, Groningen, The Netherlands
Abstract Body:
The development of integrase (IN) inhibitor resistance during dolutegravir (DTG) containing cART is exceedingly rare. This high genetic resistance barrier may make DTG suitable as maintenance monotherapy. We hypothesized that DTG monotherapy is non-inferior to cART in maintaining viral suppression.
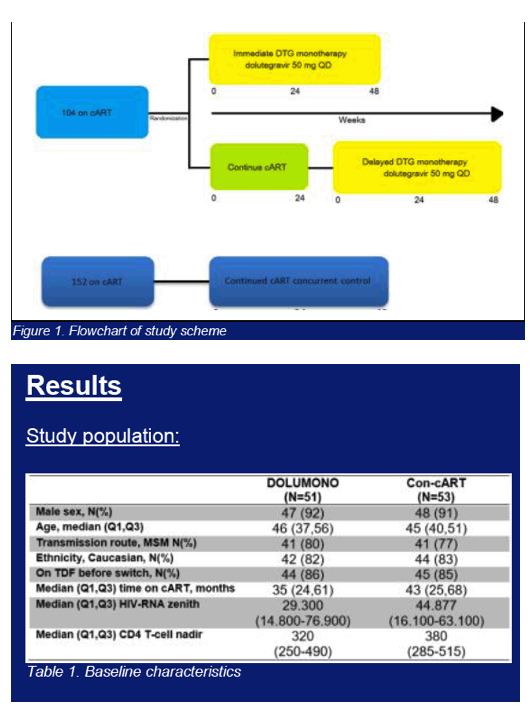
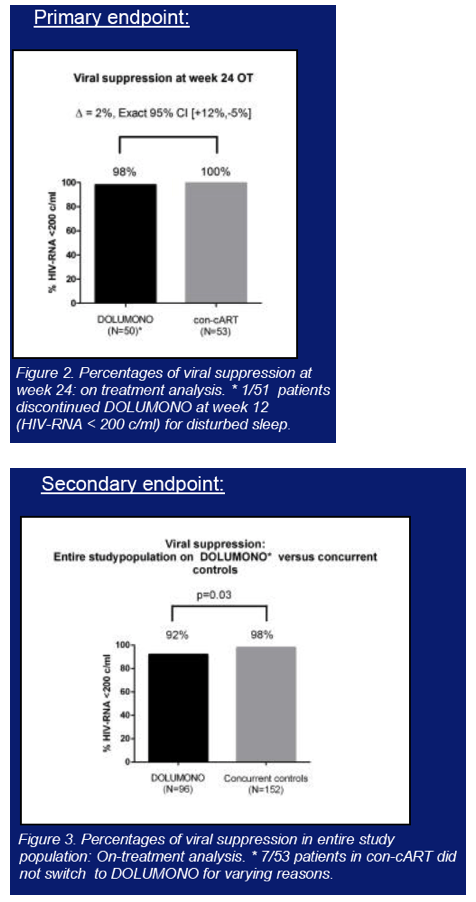
Multicenter randomized trial comparing DTG 50mg QD (DOLUMONO) with continued cART(con-cART). Pts included were HIV-1+ and on cART with a viral load (VL)<50c/ml for >6months, a CD4 nadir >200cells/ul, a pre-cART peak VL<100.000c/ml and no history of virological failure (VF). 24wks after randomization, the con-cART group switched to DOLUMONO as well (delayed switch), fig1. The primary endpoint was the on-treatment proportion of pts with a VL<200c/ml at W24. Assuming 95% viral suppression, 104 pts were needed for a study with β=80%, δ=-0.12, and α=0.025. Secondary endpoints were VL<200c/ml and <50c/ml at W24 and W48 in all pts on DOLUMONO (immediate+delayed switch group combined). Due to the 'W24 delayed switch' study design, no randomized con-cART control group is available to compare the W48 DTG monotherapy results with. Therefore, a concurrent control group of 152 pts on cART was included (fig1). These pts fulfilled the same in -and exclusion criteria but continued their cART. NCT02401828.
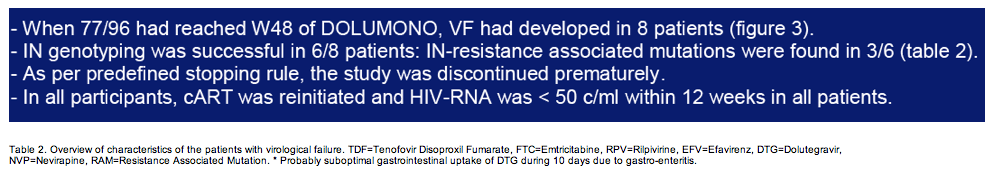
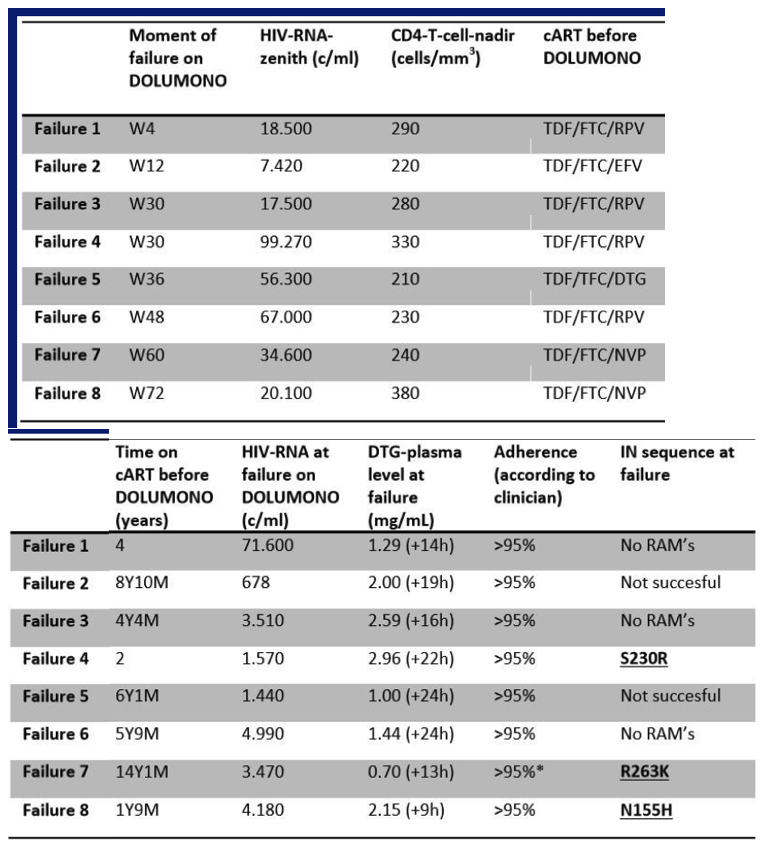
104 pts were included and on cART for 40 months with CD4 nadir of 340cells/ul. One pt discontinued DTG at W12 for adverse events. At W24, DOLUMONO was non-inferior to con-cART: VL<200c/ml in 49/50 vs 53/53 (Δ2%, Exact 95%CI +12% to -5%) with no IN resistance in the single VF. Also 46 of 53 pts randomized to con-cART switched to DOLUMONO 24 weeks after randomization. Consequently, a total of 96 pts received DTG monotherapy, of whom 94 have reached W24. 92/94 had a VL<200c/ml with no resistance in the 2 VF. However, when 77 of the 96 pts had reached W48 of monotherapy, VF had developed in 8 (2 before W24, 6 after W24). IN genotyping was successful in 6 and resistance found in 3: the 155H and 263K in 1 pt each and the 230R, a mutation not previously described during DTG therapy, in 1 pt. As per predefined stopping rule, this led to the premature study discontinuation. In the concurrent control group on cART, VF was observed significantly less (3/152 vs 8/96, p=0.03).
Although DTG monotherapy was non-inferior to cART at W24, VF continued to occur after W24 and led to DTG resistance in 3. The genetic barrier of DTG monotherapy is insufficient to allow for maintenance monotherapy.


|
| |
|
 |
 |
|
|