 |
 |
 |
| |
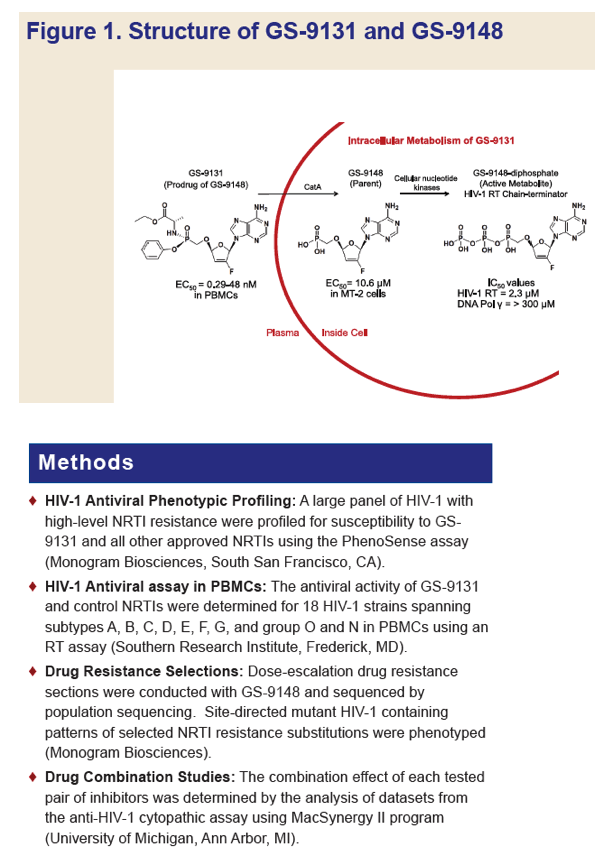
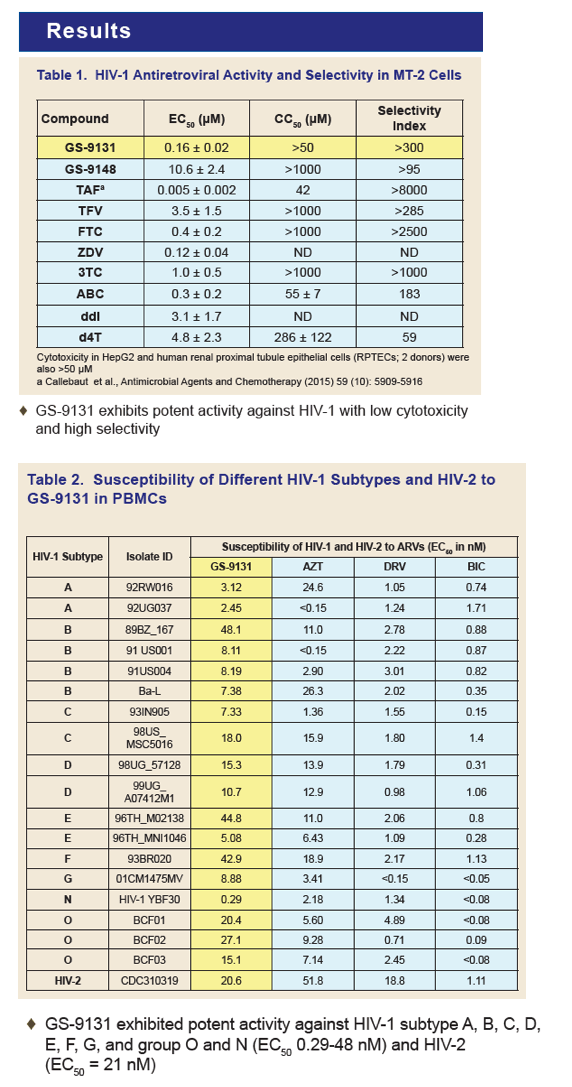
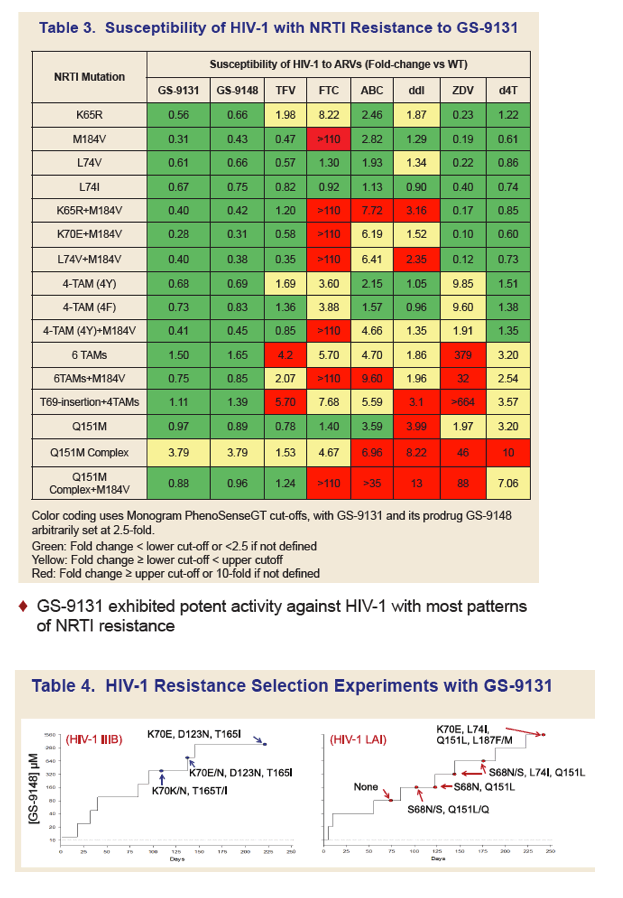
GS-9131 is a Novel NRTI with Activity Against NRTI-Resistant HIV-1
|
| |
| |
Download the PDF here
Reported by Jules Levin
CROI 2017 Feb 14-16 Seattle, WA
Kirsten White*, Nicolas Margot, Kirsten Stray, Helen Yu, George Stepan, Constantine G. Boojamra, Richard Mackman, Adrian Ray, Michael D. Miller, and Tomas Cihlar
Gilead Sciences, Foster City, CA
Novel Nucleotide Human Immunodeficiency Virus Reverse Transcriptase Inhibitor GS-9148 with a Low Nephrotoxic Potential: Characterization of Renal Transport and Accumulation http://aac.asm.org/content/53/1/150.full
In conclusion, we have established that, similar to acyclic nucleotide analogs, GS-9148 is a substrate for renal transporters hOAT1, hOAT3, and MRP4. However, the specific kinetics of these interactions result in reduced levels of active renal accumulation of GS-9148 relative to acyclic nucleotides, a difference observed both in the in vitro and in vivo models. Limited renal accumulation, coupled with low intrinsic cytotoxicity and expected reduced plasma exposure, suggests a low potential of GS-9148 to cause renal tubular dysfunction in patients treated with its prodrug, GS-9131.Nucleotide analogs carrying a negative charge under physiological conditions are substrates for the major renal organic anion transporters hOAT1 (3, 15), hOAT3 (31), and MRP4 (4, 16, 25). Results from prior in vitro and in vivo studies demonstrated that the efficient transport of antiviral acyclic nucleotides, primarily by hOAT1, mediates their selective renal accumulation that may, depending on intrinsic cytotoxicity and dose, lead to nephrotoxicity (3). The present study generated several lines of evidence indicating that GS-9148, compared to the characterized acyclic nucleotides, is less likely to accumulate in renal proximal tubule cells.
The interaction of GS-9148 with both hOAT1 and hOAT3 is less effective than cidofovir, adefovir, and tenofovir. Specifically, GS-9148 exhibits 5- to 10-fold lower affinity (Km) for hOAT1 and 60- to 100-fold lower overall transport efficiency (Vmax/Km) by the transporter compared to acyclic nucleotides. Although GS-9148 shows similar affinity for hOAT3 as adefovir and tenofovir, its transport efficiency is three- to fourfold lower than that of the acyclic nucleotides. The potential for nephrotoxicity is defined by both the propensity to accumulate in proximal tubule cells and the intrinsic cytotoxicity of a given nucleotide. Although cidofovir, adefovir, and tenofovir show similar high efficiencies of transport by the major renal transporter hOAT1, they differ substantially in their in vitro cytotoxicities, and this appears to correlate with their different in vivo nephrotoxicity potentials.Similar to tenofovir, GS-9148 at concentrations exceeding 1 mM exhibits minimal in vitro cytotoxicity in primary human renal proximal tubule cells from multiple donors as well as in other cell types.Inhibition of mitochondrial DNA polymerase γ by active metabolites of nucleoside analogs has been implicated in various clinical adverse effects (18, 33), and several authors suggested that it may also represent the underlying mechanism for the nucleotide-associated renal toxicity (6, 30). We have previously shown that the active diphosphate metabolite of GS-9148 is a poor inhibitor of mitochondrial DNA polymerase γ and neither GS-9148 nor its various prodrugs, including GS-9131, affect the levels of mitochondrial DNA in cells treated with supratherapeutic concentrations (5). Therefore, it is unlikely that the clinical administration of GS-9131 would result in mitochondrial toxicity in either kidneys or other organs or tissues.In addition to data from in vitro renal transport and accumulation studies, tissue distribution and pharmacokinetic studies conducted with radiolabeled GS-9131 in dogs illustrate that GS-9148 accumulates to a lesser extent in kidneys in vivo and has a lower proportion of material cleared by active tubular secretion than acyclic nucleotide analogs.The estimated CLr based on the amount of intact GS-9148 observed in the urine over 24 h was 0.47 liters/h/kg. This value is only slightly above the glomerular filtration rate (0.37 liters/h/kg) and markedly below renal blood flow (1.3 liters/h/kg) in the dog (12), suggesting only a limited contribution of the net active tubular secretion to the overall CLr of GS-9148. In fact, the predominant mode of GS-9148 renal elimination in the dog is likely passive filtration at the glomerulus. This is in contrast to acyclic nucleotide analogs, for which CLr has been measured to be in excess of twofold above the glomerular filtration rate in various species, including human, (1, 7, 8, 11). The reduced renal accumulation and elimination by active tubular secretion of GS-9148 are consistent with the lack of renal findings in Sprague-Dawley rats, beagle dogs, and cynomolgus monkeys treated orally with GS-9131 for 28 days at doses up to 300, 20, and 30 mg/kg/day, respectively.
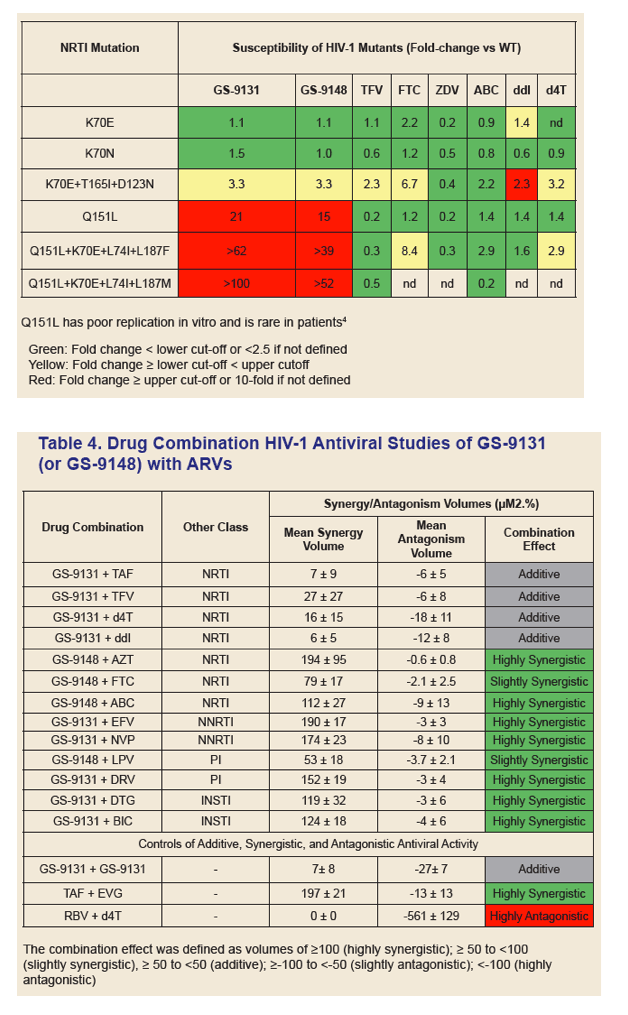

|
| |
|
 |
 |
|
|