 |
 |
 |
| |
Antiviral Activity of EFdA [MK-8591] Against NRTI-Sensitive and -Resistant Strains of HIV-2
|
| |
| |
"potential pre-exposure prophylaxis agent to prevent HIV transmission in women and their infants......MK-8591 (or EFdA) is a NRTI with very potent in vitro activity against HIV....Viral suppression was maintained for at least 7 days after the last dose. This study provides proof of concept for a once weekly oral dosing strategy of MK-8591....."Our data demonstrated that EFdA efficiently prevents both vaginal and oral HIV transmission. Together with EFdA's relatively low toxicity and high potency against drug-resistant HIV strains, these data support further clinical development of EFdA as a potential pre-exposure prophylaxis (PrEP) agent to prevent HIV transmission in women and their infants."
EFdA / MK-8591 - HIV pre-exposure prophylaxis for women and infants prevents vaginal and oral HIV transmission in a preclinical model of HIV infection - (08/05/16)
Pre-exposure Prophylaxis with EFdA Offers Strong Protection against High Dose Mucosal HIV Challenges - [Long-Acting New NRTI for Treatment & PrEP] - (10/24/16)
----------------------
Reported by Jules Levin
CROI 2017 Feb 14-16 Seattle, WA
Vincent H. Wu1, Robert A. Smith1, Sara Masoum1, Dana N. Raugi1, Selly Ba2, Moussa Seydi2, Jay Grobler3, and Geoffrey S. Gottlieb1,4
for the University of Washington-Dakar HIV-2 Study Group
1Department of Medicine, Division of Allergy and Infectious Diseases and 4Department of Global Health, University of Washington, Seattle, Washington, USA
2Clinique des Maladies Infectieuses Ibrahima DIOP Mar, Centre Hospitalier Universitaire de Fann, Universite Cheikh Anta Diop de Dakar, Dakar, Senegal
3Merck & Co., Inc., West Point, Pennsylvania, USA
Abstract Body:
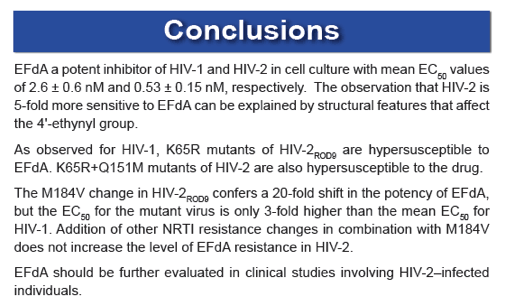
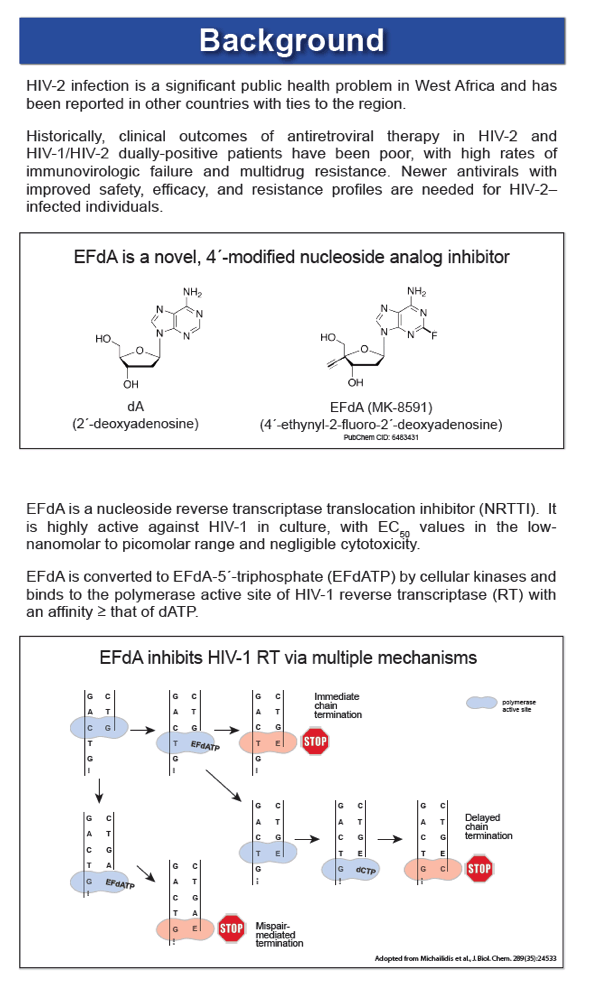
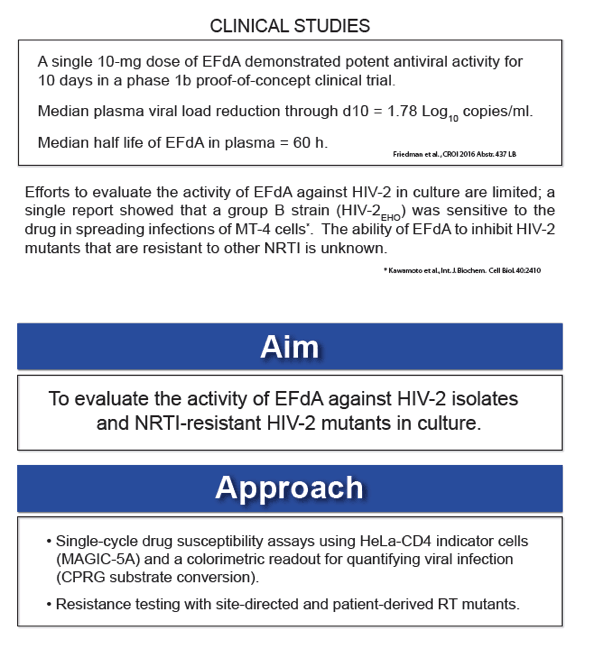
EFdA (4'-ethynyl-2-fluoro-2'-deoxyadenosine; MK-8591; Merck & Co.) is an investigational NRTI that blocks HIV-1 replication in culture with 50% effective concentrations (EC50) in the low-nanomolar to picomolar range. EFdA is highly active against HIV-1 and SIV in humanized mice and rhesus macaques, respectively, and a single 10-mg dose of EFdA demonstrated potent antiviral activity for 10 days in a phase 1b proof-of-concept clinical trial. However, studies evaluating the activity of the EFdA against HIV-2 are lacking, and the ability of the drug to inhibit NRTI-resistant mutants of HIV-2 is unknown.
HIV-1 and HIV-2 isolates from antiretroviral-naïve individuals were tested against EFdA in single-cycle infections of MAGIC-5A cells. Site-directed mutants of HIV-2 reverse transcriptase (RT) were generated in a full-length plasmid clone (pROD9) and were evaluated for EFdA resistance in the single-cycle assay. 50% cytotoxic concentrations (CC50) for EFdA were determined using a CellTiter-Glo® luminescence kit.
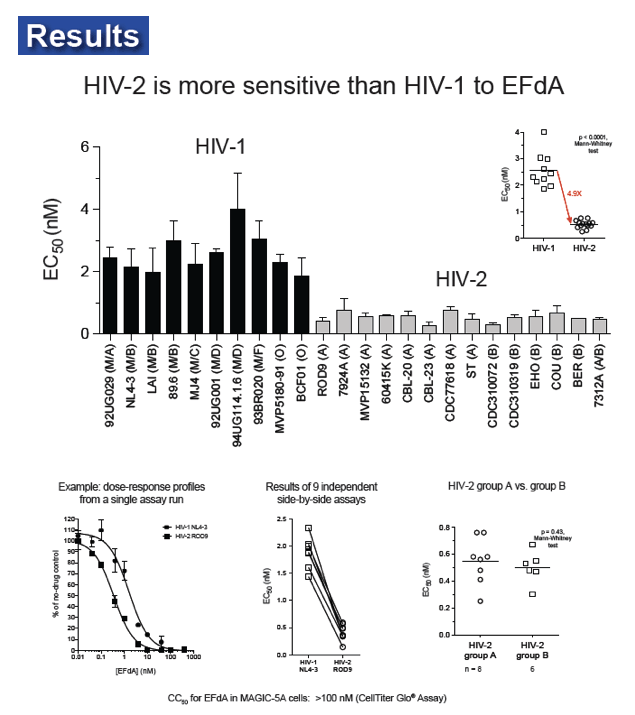
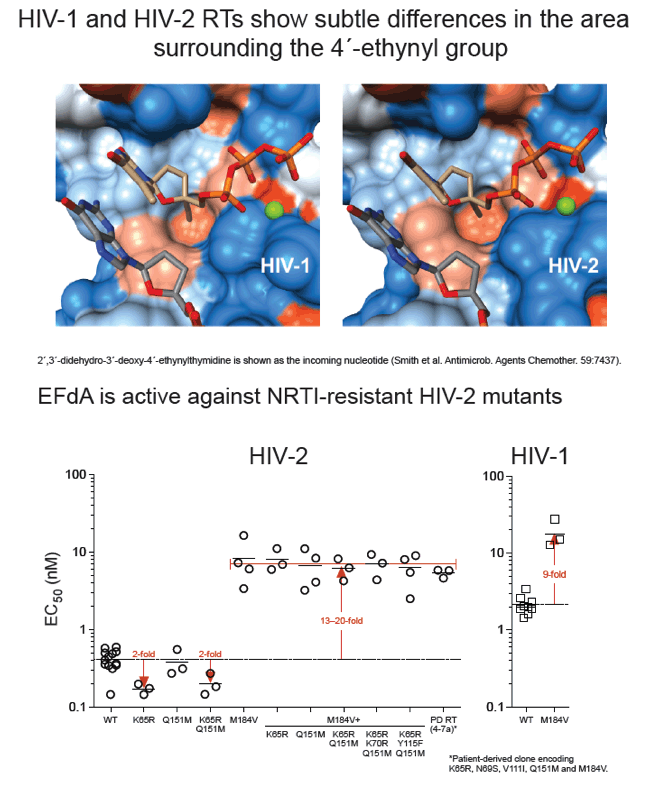
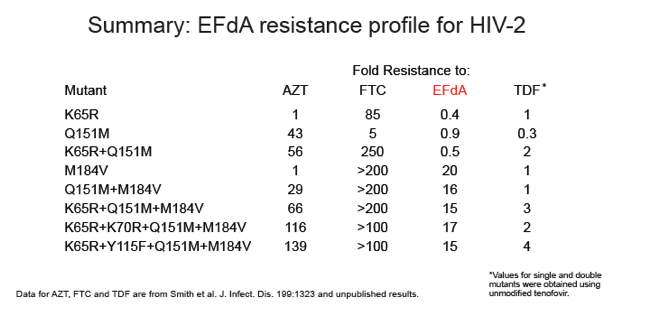
EFdA inhibited HIV-2 infection of MAGIC-5A cells with mean EC50 values (± SD) of 0.58 ± 0.13 nM for 6 group A isolates and 0.55 ± 0.16 nM for 6 group B isolates (range = 0.34-0.83 nM for all 12 HIV-2 strains tested). In contrast, the mean EC50 for 6 HIV-1 isolates, including group M subtype A, B, C and D strains and the group O isolate MVP5180-91, was 2.0 ± 0.43 nM (range = 1.29-2.54 nM; p < 0.0001 for HIV-1 vs. HIV-2, Mann-Whitney test). In spreading infections of CEMss cells, EC50 values for HIV-2 ROD9 and HIV-1 NL4-3 were 38 and 120 pM, respectively. EFdA was fully active against HIV-2 RT mutants K65R and Q151M (EC50 = 0.17 ± 0.04 nM and 0.31 ± 0.05 nM, respectively), whereas the M184V variant was 10-fold resistant to the drug. Similar levels of resistance (12-16-fold) were seen for HIV-2 mutants that harbored M184V plus one or more additional NRTI resistance-associated changes in RT, including a patient-derived clone encoding K65R+N69S+V111I+Q151M+M184V. The CC50 for EFdA in MAGIC-5A cells was >100 nM.
EFdA is the most potent inhibitor of HIV-2 replication described to date and is more active against HIV-2 than against HIV-1 in culture. EFdA also inhibits multi-NRTI-resistant HIV-2 mutants with single-cycle EC50 values ≤ 10 nM. These data indicate that EFdA should be evaluated in clinical studies involving HIV-2-infected individuals.
|
| |
|
 |
 |
|
|