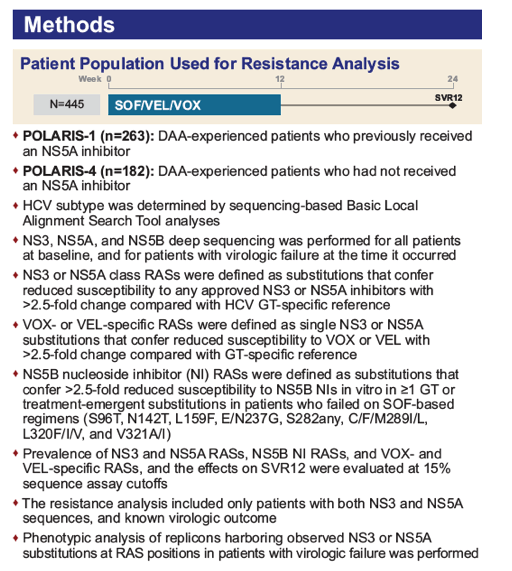
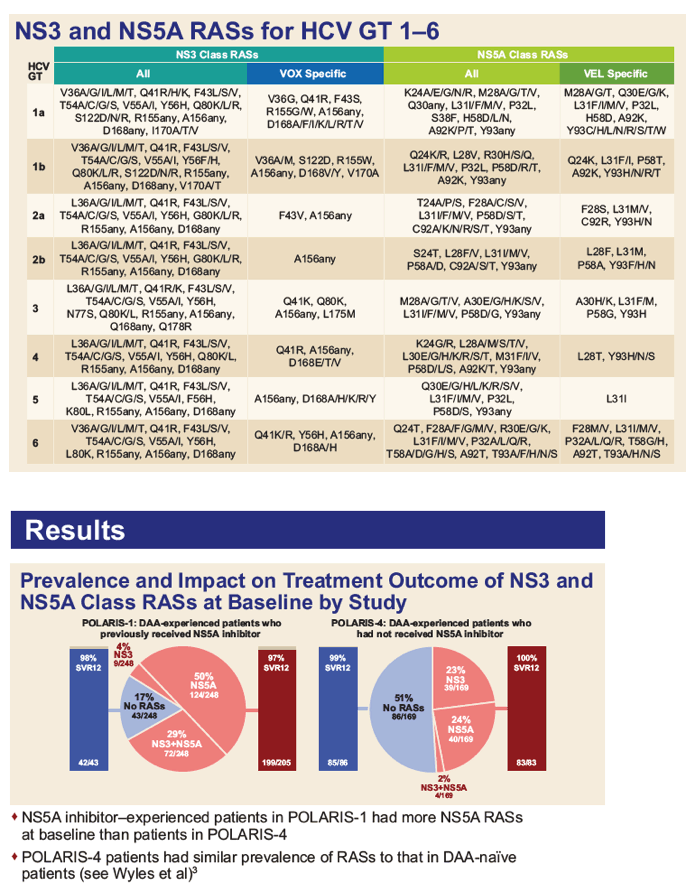
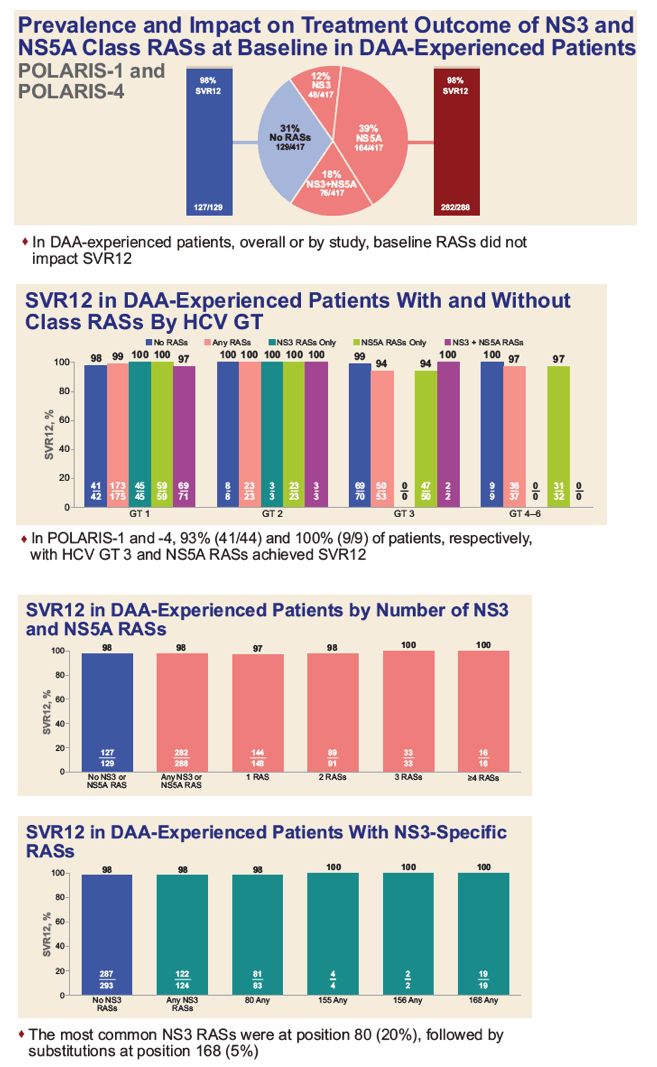
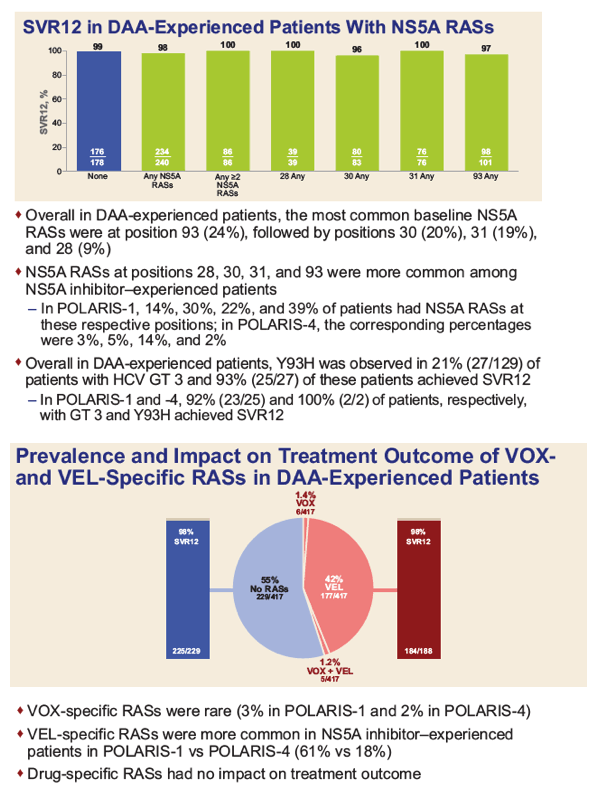
 |
 |
 |
| |
No Impact of RASs on the High Efficacy of SOF/VEL/VOX for 12 Weeks in DAA-Experienced Patients:
an Integrated Resistance Analysis of the POLARIS-1 and POLARIS-4 Studies
|
| |
| |
Reported by Jules Levin
EASL 2017 April 19-23 Amsterdam Netherlands
Christoph Sarrazin,1 Curtis L. Cooper,2 Michael P. Manns,3 Rajender Reddy,4 Kris Kowdley,5 Hadas Dvory-Sobol,6 Evguenia Svarovskia,6 Ross Martin,6 Gregory Camus,6
Brian P. Doehle,6 Luisa M. Stamm,6 Robert H. Hyland,6 Diana M. Brainard,6 Hongmei Mo,6 Stuart C. Gordon,7 Marc Bourliere,8 Stefan Zeuzem,9 Steven L. Flamm10
1Medizinische Klinik 1, Universitätsklinikum Frankfurt, and St. Josefs-Hospital Wiesbaden GmbH, Germany; 2University of Ottawa, Ontario, Canada; 3Medizinische Hochschule Hannover, Germany; 4University of Pennsylvania, Philadelphia, USA;
5Swedish Medical Center, Seattle, Washington, USA; 6Gilead Sciences, Inc., Foster City, California, USA; 7Henry Ford Health System, Detroit, Michigan, USA; 8Hôpital Saint Joseph Marseilles, France; 9Medizinische Klinik 1, Universitätsklinikum Frankfurt; 10Northwestern University, Chicago, Illinois, USA
EASL2017: No Impact of RASs on the High Efficacy of SOF/VEL/VOX for 8 Weeks in DAA-Naïve Patients: an Integrated Resistance Analysis of the POLARIS-2 and POLARIS-3 Studies - (04/20/17)
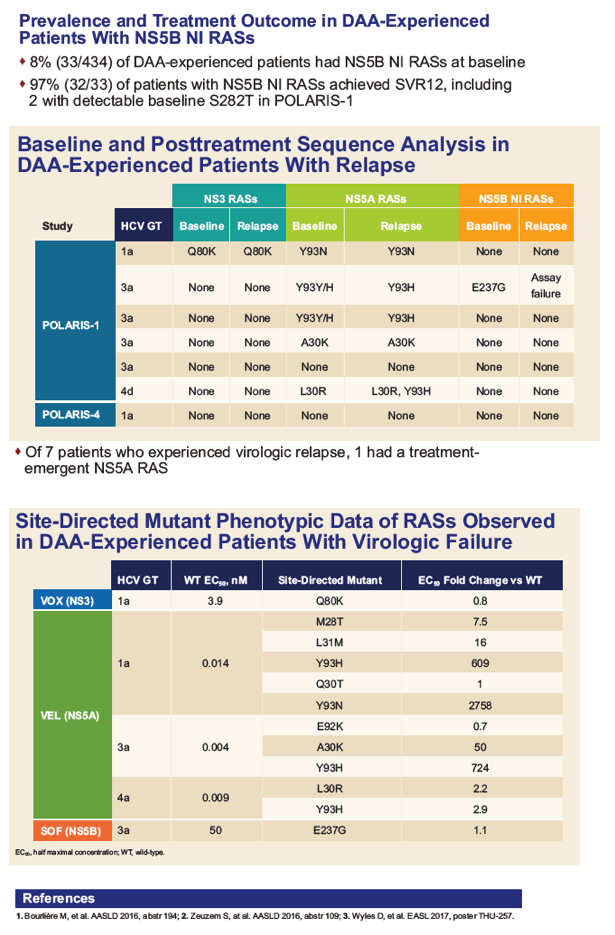
AASLD: A Randomized, Controlled, Phase 3 Trial of Sofosbuvir/Velpatasvir/Voxilaprevir or Sofosbuvir/Velpatasvir for 12 Weeks in Direct-Acting Antiviral-Experienced Patients With Genotype 1-6 HCV Infection: The POLARIS-4 Study - (11/14/16)
AASLD: Sofosbuvir/Velpatasvir/Voxilaprevir for 12 Weeks as a Salvage Regimen in NS5A Inhibitor-Experienced Patients With Genotype 1-6 Infection: The Phase 3 POLARIS-1 Study - (11/15/16)

|
| |
|
 |
 |
|
|