 |
 |
 |
| |
Safety and Efficacy of Sofosbuvir and Velpatasvir with or without Ribavirin for the Treatment of HCV Genotype 1-6: Results of the HCV-TARGET Study
|
| |
| |
Reported by Jules Levin
EASL 2017 April 19-23 Amsterdam Netherlands
Khalili, M; Welzel, TM; Terrault, N; Lim, J; Sridhar, A; Lutchman, G; Nelson, D; Borg, B; Lok AS; Ramani, A; Reau, N; Vainorius, M; Fried, MW; Landis, C
EASL:No Impact of RASs on the High Efficacy of SOF/VEL/VOX for 12 Weeks in DAA-Experienced Patients: an Integrated Resistance Analysis of the POLARIS-1 and POLARIS-4 Studies - (04/26/17)
EASL: SOF/VEL/VOX Results in High SVR12 Rates When Administered for 12 Weeks in DAA-Experienced Patients or for 8 Weeks in DAA-Naïve Patients: an Integrated Analysis of the POLARIS-1, POLARIS-2, POLARIS-3, and POLARIS-4 Studies - (04/25/17)
EASL: SOF/VEL/VOX for 8 or 12 Weeks Is Well Tolerated and Results in High SVR12 Rates in Patients Receiving Opioid Substitution Therapy - (04/24/17)
EASL: The Safety and Tolerability of SOF/VEL/VOX for 8 or 12 Weeks in >1000 Patients Treated in the POLARIS-1, POLARIS-2, POLARIS-3, and POLARIS-4 Studies: an Integrated Analysi - (04/26/17)
EASL: Utilization of DAA therapies ledipasvir/sofosbuvir and sofosbuvir/velpatasvir in patients with genotype 1 HCV: Real-world experience from the TRIO Network. - (04/26/17)
EASL: Sofosbuvir/velpatasvir compared to existing Standards of Care in GT2-6 HCV Real-world experience from the TRIO Network - (04/24/17)
Sofosbuvir and Velpatasvir for the Treatment of HCV in Patients Coinfected with HIV-1: an Open-Label, Phase 3 Study - (04/12/17)
EASL: No Impact of RASs on the High Efficacy of SOF/VEL/VOX for 8 Weeks in DAA-Naïve Patients: an Integrated Resistance Analysis of the POLARIS-2 and POLARIS-3 Studies - (04/20/17)
EASL: Treatment With SOF/VEL or SOF/VEL/VOX Is Well Tolerated and Results in High SVR12 in Genotype 1-6 HCV-Infected Patients With Minimal Fibrosis: a Retrospective Analysis of the ASTRAL and POLARIS Clinical Studies - (04/21/17)
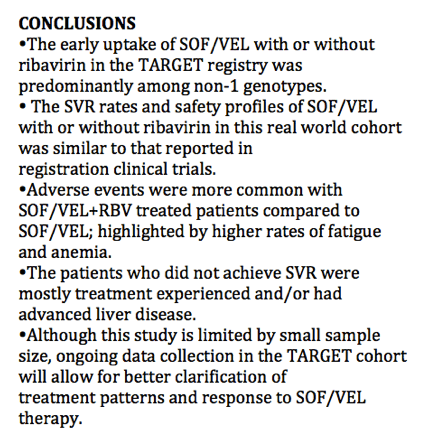
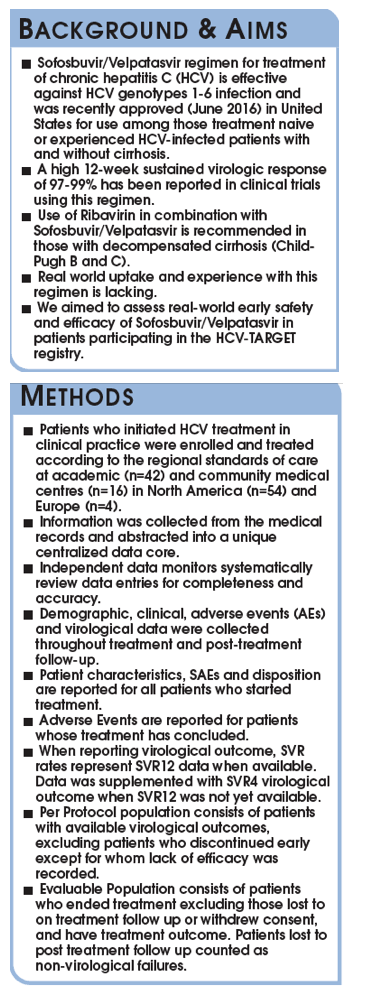
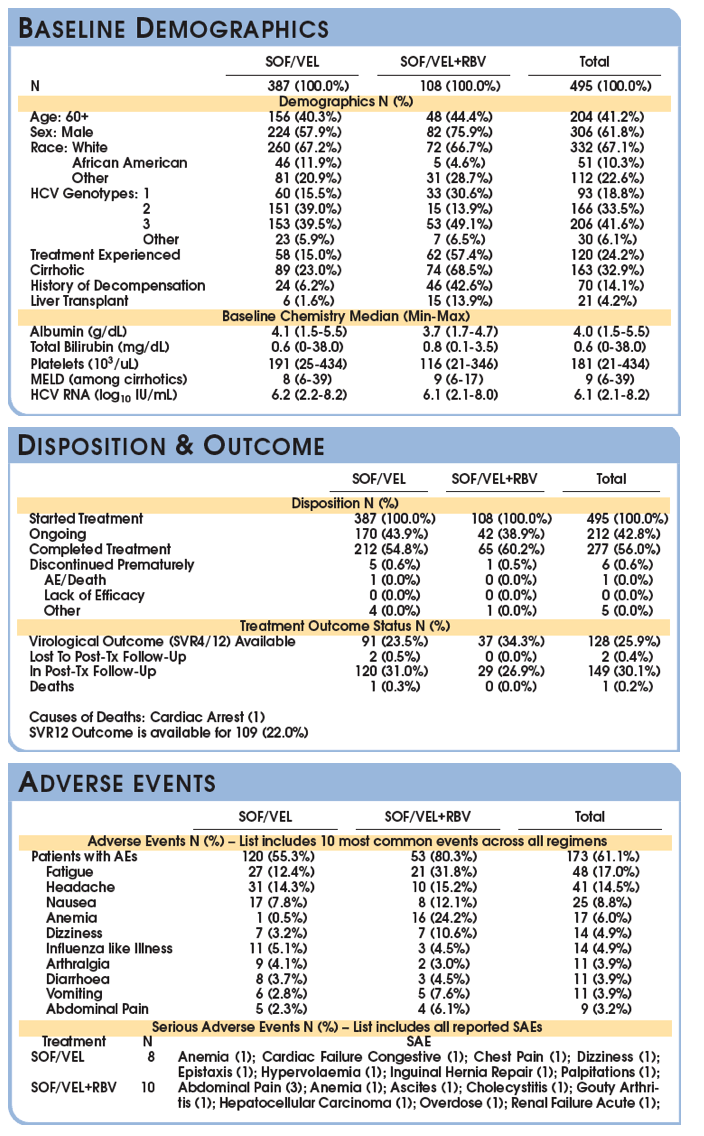
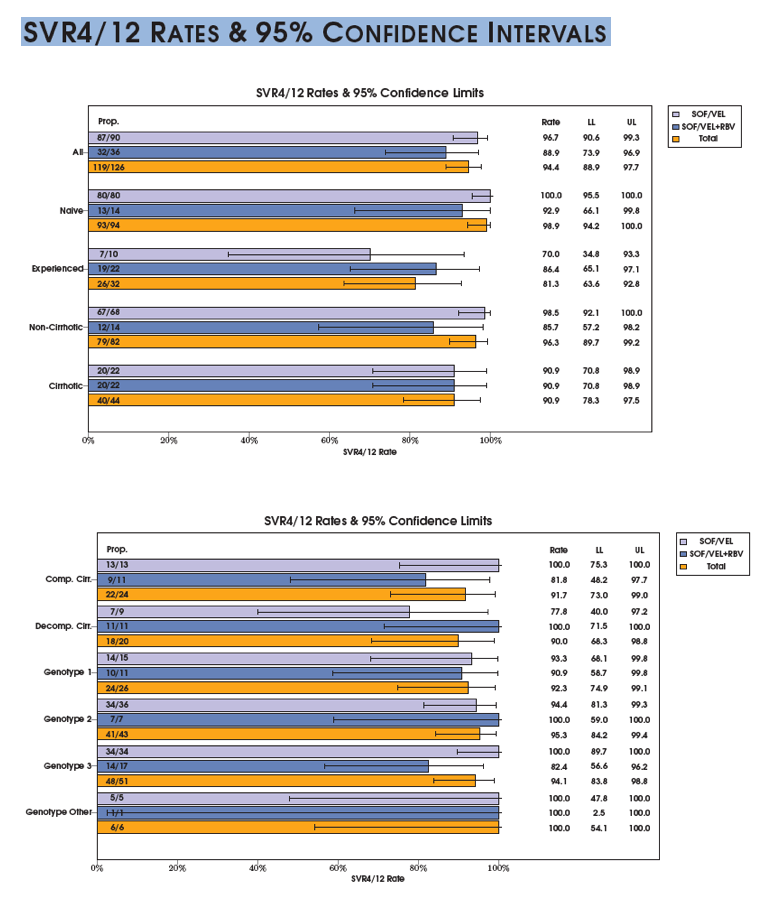
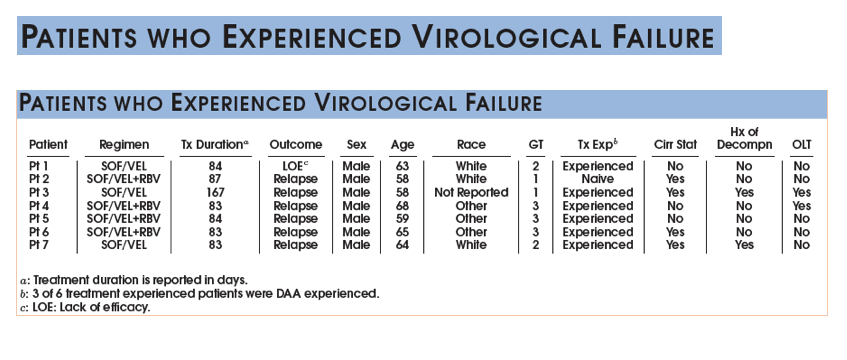
SVR is presented among the Per Protocol population (patients with available virological outcomes, excluding patients discontinued early except for whom lack of efficacy was recorded.)
The SVR4/12 rate in the evaluable population is (121/128) 94.5% with a 95% CI of (89.1, 97.8).
|
| |
|
 |
 |
|
|