| |
New HIV Drugs - Heavily Treated with Multi-Drug Resistance
| | | | |
Download the PDF here
http://www.natap.org/2017/HIV/120817_05.htm
--------------------------------------
A decade of HIV-1 drug resistance in the United States: trends and characteristics in a large protease/reverse transcriptase and co-receptor tropism database from 2003 to 2012
Antiviral Therapy 2014 - Agnes C Paquet1, Owen D Solberg1, Laura A Napolitano1, Joseph M Volpe1, Charles Walworth1, Jeannette M Whitcomb1, Christos J Petropoulos1, Mojgan Haddad1,* 1Monogram Biosciences, South San Francisco, CA, USA
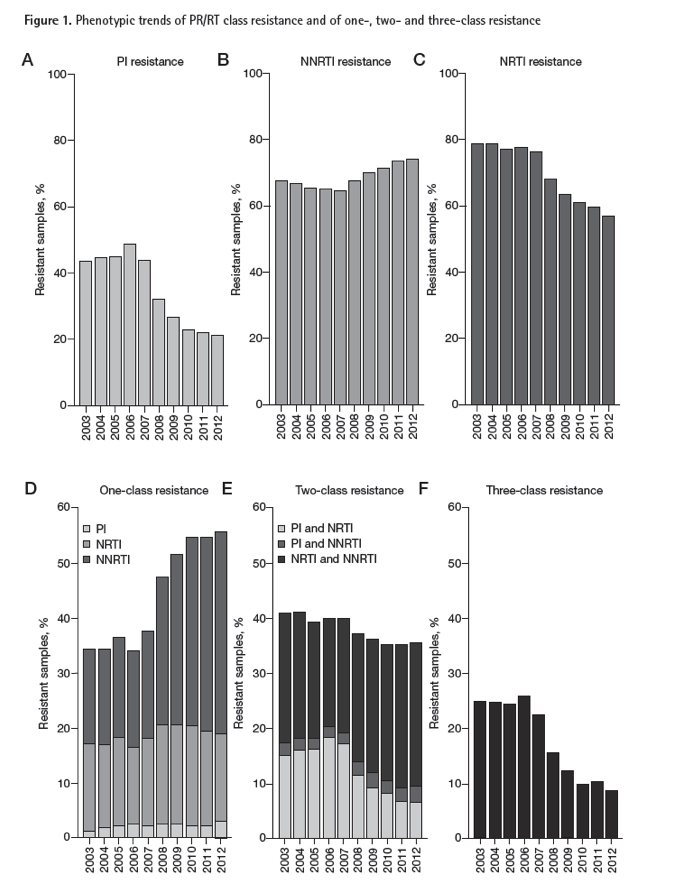
"routine phenotypic and genotypic resistance testing to protease inhibitors (PIs), nucleoside reverse transcriptase inhibitors (NRTIs) and non-nucleoside reverse transcriptase inhibitors (NNRTIs), as well as for tropism determination were investigated.....Results: Within 62,397 resistant viruses reported from 2003 to 2012, we observed a decreasing trend in the prevalence of three-class resistance (from 25% to 9%) driven by decreased resistance to PIs (43% to 21%) and NRTIs (79% to 57%), while observing a slight increase in NNRTI resistance (68% to 75%). The prevalence of CXCR4-mediated entry among tropism testing samples (n=52,945) declined over time from 47% in 2007 to 40% in 2012. A higher proportion of CXCR4-tropic viruses was observed within samples with three-class resistance (50%) compared with the group with no resistance (36%). Conclusions: Decreased prevalence of three-class resistance and increased prevalence of one-class resistance was observed within samples reported between 2003 and 2012. The fraction of CXCR4-tropic viruses has decreased over time; however, CXCR4 usage was more prevalent among multi-class-resistant samples, which may be due to the more advanced disease stage of treatment-experienced patients. These trends have important implications for clinical practice and future drug discovery and development."
Attached pdf
----------------------------------
New HIV Drugs - Heavily Treated with Multi-Drug resistance
http://www.natap.org/2017/HIV/120817_05.htm
Theratechnologies Announces Decision by the FDA to Extend the Ibalizumab Review Period to April 3, 2018 - (11/16/17)
In a notice received today by TaiMed from the FDA, the Prescription Drug User Fee Act ("PDUFA") target action date has been extended to April 3, 2018. The three-month extension period is the FDA's standard extension period.
FDA Ibalizumab Expected Approval Date / 2 New HIV Drugs for Heavily Treated with Multi-Drug resistance - (12/08/17)
♦ Phase 3 Study of Fostemsavir in Heavily Treatment-Experienced HIV-1-Infected Participants: Day 8 and Week 24 Primary Efficacy and Safety Results (BRIGHTE Study, Formerly 205888/AI438-047) - (10/30/17)
♦ LONG-ACTING IBALIZUMAB SUSCEPTIBILITY IN MULTI-DRUG RESISTANT HIV PATIENTS - (10/12/17)
♦ CROI: PRO 140 Single-Agent Maintenance Therapy for HIV-1 Infection: A 2-Year Update- (03/01/17)
----------------------------
48-Week Safety and Efficacy On-Treatment Analysis of Ibalizumab in ...
www.natap.org/2017/IDWeek/IDWeek_38.htm
LONG-ACTING IBALIZUMAB SUSCEPTIBILITY IN MULTI-DRUG...
www.natap.org/2017/IAS/IAS_150.htm
Expanded Access Program for ibalizumab in Resistant HIV-1 - NATAP
www.natap.org/2017/HIV/051717_06.htm
LONG-ACTING IBALIZUMAB IN PATIENTS WITH MULTI-DRUG ...
www.natap.org/2017/CROI/croi_20.htm
Theratechnologies Announces New Data from the Pivotal Phase III ...
www.natap.org/2017/CROI/croi_19.htm
Monoclonal Antibody Suppresses HIV in Half With Multidrug-Resistant ...
www.natap.org/2017/CROI/croi_07.htm
for Ibalizumab Presented at IDWeek 2017 - NATAP
www.natap.org/2017/HIV/100417_01.htm
|
| | | | | | |
|