| |
Psychiatric Symptoms in Patients Receiving Dolutegravir
|
| |
| |
"In those patients who developed depression whose psychiatric history was known (34/100),
approximately 85% had a history of depression before drug initiation. An absence of past history of depressive symptoms was only identified in 3 cases.
Among DTG-treated patients who experienced PSs, few were considered grade 3 to 4 intensity or reported as serious, and most DTG-treated patients (95%) who experienced suicidality had a past history of psychiatric conditions
Of the 20 cases of suicidality in comparator-treated patients, 16 (80%) had a psychiatric history at baseline, with a similar pattern as observed for DTG, 2 had socioeconomic triggers, and 2 had no obvious underlying risk factors. The DTG-treated patient who completed suicide was male, of unknown age, and had a previous medical history of bipolar disorder requiring
treatment, suicidal ideation, and suicide attempt. The RAL-treated patient who completed suicide was also male, aged 22 years, and had no reported underlying risk factors or triggers. Neither case of completed suicide was considered drug related."
Among cases of suicidality with reported TTO (~50%), suicidality typically occurred within 1 month of starting treatment with DTG or ABC/DTG/3TC. In the 40% of suicidality cases for which past psychiatric history was available, 16 of 20 patients had a psychiatric condition before starting treatment. Five patients completed suicide (DTG, n=4; ABC/DTG/3TC, n=1). Four of the 5 cases occurred within 6 months of commencing treatment (Supplementary Table 3). Three of these patients had a history of depression and 1 previously attempted suicide. In the case for which psychiatric history was not available, there was evidence suggesting drug abuse and psychiatric instability when the patient started treatment. The final case involved a 55-year-old man living with HIV for approximately 30 years who had multiple co-morbidities; the suicide was considered unrelated to DTG."
Download the PDF here
JAIDS -Journal of Acquired Immune Deficiency Syndromes Publish Ahead of Print Jan 20 2017
Anna Fettiplace, PhD, MBChB,1 Chris Stainsby, BSc Hons,1 Alan Winston, MD,2 Naomi Givens,MSc,1 Sarah Puccini, BSc Hons,1 Vani Vannappagari, PhD,3Ricky Hsu, MD,4 Jennifer Fusco,BS,5 Romina Quercia, MD, PhD,3 Michael Aboud, MBChB, MRCP,3 Lloyd Curtis, MA, MRCP1
1GlaxoSmithKline, London, UK; 2Imperial College, London, UK; 3ViiV Healthcare, ResearchTriangle Park, NC, USA; 4NYU Langone Medical Center, New York, NY, USA; 5Epividian Inc.,Research Triangle Park, NC, USA
ABSTRACT
Introduction: Psychiatric symptoms are reported to occur frequently in people living with HIVand may be associated with specific antiretrovirals. We analysed psychiatric symptomsobserved with dolutegravir and other frequently prescribed anchor drugs.
Methods: Selected psychiatric symptoms (insomnia, anxiety, depression, and suicidality)occurring in HIV-positive patients during dolutegravir treatment across 5 randomized clinicaltrials (3 double-blind), in the Observational Pharmaco-Epidemiology Research & Analysis(OPERA®) cohort, and among cases spontaneously reported to ViiV Healthcare were analysed.
Results: In clinical trials, psychiatric symptoms were reported at low and similar rates inpatients receiving dolutegravir or comparators (atazanavir, darunavir, efavirenz, or raltegravir).Insomnia was most commonly reported. The highest rates were observed in SINGLE(dolutegravir 17%, efavirenz 12%), with consistently lower rates in the other trials (dolutegravir:3%-8% versus comparator: 3%-7%). More efavirenz-treated patients withdrew because ofpsychiatric symptoms than patients treated with other anchor drugs. In OPERA, history ofpsychiatric symptoms at baseline was lowest in efavirenz-treated patients compared withpatients treated with dolutegravir, raltegravir, or darunavir. Despite baseline differences,prevalence and incidence during treatment were similar across the 4 anchor drugs. Withdrawalrates for psychiatric symptoms were lowest for dolutegravir (0%-0.6%) and highest forraltegravir (0%-2.5%). Spontaneously reported events were similar in nature to clinical trialdata.
Conclusions: Analysis of 3 different data sources shows that, similar to other frequentlyprescribed anchor drugs to treat HIV infection, psychiatric symptoms are also reported in DTG-treatedpatients. These events are reported with low frequency and rarely necessitate DTGdiscontinuation.
from Introduction excerpt:
PSs are substantially more frequent among persons living with HIV (PLWH; anxiety28%, depression up to 48%, insomnia 29% to 73%)11-15 compared with the general population(anxiety 7.3%, depression 5% to 10%, insomnia 3.6% to 18%).11,12,15-17 The rate of suicide for
PLWH has been reported to be up to 8 times higher than that of the general population.18,19
Asystematic review of studies published between 1989 and 2008 showed a prevalence of 27%(range 4% to 78%) for suicidal ideation among PLWH.20
The etiology is likelymultifactorial, including immune activation due to HIV disease, antiretroviral toxicities, stigmafrom living with HIV, and lifestyle factors, such as drug and alcohol use, which may be higher inPLWH compared to control populations.15,21-23 Although antiretroviral therapy improves survivaland reduces morbidity in PLWH, PSs may be associated with certain antiretroviral agents.10,24
High background rates ofpsychiatric conditions among PLWH and the likelymultifactorial pathogenesis make it challenging to assess the relationship between PSs andspecific antiretroviral therapies. The objectives of this analysis are to provide insight into thefrequency and characteristics of PSs that have been reported in patients treated with DTG-basedregimens using new data sources, including aggregated data from ViiV Healthcareclinical trials, the Observational Pharmaco-Epidemiology Research & Analysis (OPERA®)25
from Discussion section:The analysis of 3 different data sources shows that, similar to other frequently prescribed
anchor drugs to treat HIV infection, PSs are also reported in DTG-treated patients. These
events are reported with low frequency and most are generally mild to moderate in intensity,
non-serious (with the exception of suicidality), and rarely necessitate DTG discontinuation....
This analysis was conducted to characterize PSs [psych symptoms] occurring in patients during HIV-1treatment with DTG compared with other recommended anchor drugs. Psychiatric symptomsare more prevalent in the HIV-infected population than in the general population11 and, givenother confounders present, are difficult to associate with specific antiretroviral therapies. Due tothe nature of the data collected, this paper looks at psychiatric symptoms rather than formalpsychiatric diagnoses, and the relationship between these varies with the data source.....The individual PSs occurred at a low frequency inpatients treated with DTG in clinical trials and the OPERA cohort, and spontaneous reportingrates were low in the context of estimated patient years of exposure.....The most frequentlyreported events (i.e., insomnia, anxiety, and depression) were generally mild to moderate inintensity. Discontinuation rates due to PSs were low in DTG-treated patients in clinical trials andthe OPERA cohort, and where outcome information was available (e.g., in clinical trials) events.....typically resolved or improved on continued treatment. In spontaneously reported cases,discontinuation rates were higher, which could be influenced by the limitations of spontaneousreporting discussed below (e.g., reporting bias). The OPERA cohort data may represent thereal-world setting more accurately than data from spontaneous reporting because of the lowdiscontinuation rates and the ability to calculate incidence rates based on known denominators.None of the patients in the clinical trials were also in the OPERA cohort.....The most frequently reported drug-related PS was insomnia, occurring in 3.5% ofpatients treated with DTG in clinical trials, which led to withdrawal in only 2 patients. Insomniawas also the most common spontaneously reported PS (1.23 cases per 1,000 PY in DTG-treatedpatients, 1.11 cases per 1,000 PY in ABC/DTG/3TC−treated patients). However, in theOPERA cohort, both prevalence and new incidence of insomnia following treatment were similarto anxiety and depression for all 4 anchor drugs. The incidence of insomnia for patients treatedwith DTG was reproducibly low (3% to 8%) and similar to INSTI and protease inhibitor (PI)comparators in 4 of 5 clinical trials, with the SINGLE trial being an obvious outlier......EFV/TDF/FTC was the double-blind comparator in SINGLE, and because the adversedrug-reaction profile for EFV includes a high incidence of psychiatric events, HCPs may havemonitored patients more closely for psychiatric symptoms and been more likely to report suchevents during this trial. Patients may also have been more likely to report such symptomsknowing that there was a 50% chance that they were randomized to EFV. A similar finding waspreviously observed in the 2 double-blind, registrational clinical trials for RAL in which higherreporting rates for insomnia and abnormal dreams were observed in STARTMRK, which had anEFV comparator group, compared with BENCHMRK, which did not.32.....In the 5 clinical trials we examined, frequencies of PSs were low with DTG andsimilar to the comparator groups, with the exception of insomnia in SINGLE, and the majority ofpatients developed a single episode. Among DTG-treated patients who experienced PSs, fewwere considered grade 3 to 4 intensity or reported as serious, and most DTG-treated patients(95%) who experienced suicidality had a past history of psychiatric conditions......Using OPERA data that included 98% of all DTG data allowed PSs to be assessed inpatients in a real-world setting and allowed all drugs of interest to be compared starting from thesame date and for the same follow-up period. Data for EFV, RAL, and DRV before DTG wasintroduced in 2013 were consistent with the data set used in this study (data not shown). Theseresults support conclusions from the clinical trial data that the frequency of PSs associated withDTG was generally low and similar to that of other anchor drugs, and most similar to DRV.
Patients treated with EFV had the lowest prevalence of PSs at baseline (before medication wasinitiated), which suggests that in the real-world setting physicians may be preferentiallyprescribing INSTIs and DRV rather than EFV to treat patients with a history of psychiatricevents. Despite this potential channeling bias, the prevalence, incidence, and withdrawal ratesof PSs with DTG remained low compared with patients treated with the other anchor drugs,including EFV."
------------------------------
RESULTS section excerpts:
Insomnia was the most commonly reported PS. The highest rates were reported inSINGLE (DTG, 71/414 [17%]; EFV, 52/419 [12%]), with consistently lower rates reported in theother 4 trials (SPRING-2: DTG 6%, RAL 5%; SAILING: DTG 3%, RAL 4%; FLAMINGO: DTG
8%, DRV/r 7%; ARIA: DTG 4%, ATV/r 3%). The SINGLE study reported the highest proportionof insomnia cases considered drug related by reporting investigators (DTG, 43/71 [61%]; EFV,28/52 [54%]) and the shortest median TTO (DTG, 16.0 days [1.0-72.0]; EFV, 30 days [2.0-218.0]). Four grade 3 to 4 insomnia cases occurred in DTG-treated patients and 0 in thecomparator groups; none were considered serious (Supplementary Table 1). Two DTG-treatedpatients withdrew across trials due to insomnia compared with 4 treated with EFV/TDF/FTC in Sinlge (Table 1).
"The objectives of this analysis are to provide insight into thefrequency and characteristics of PSs that have been reported in patients treated with DTG-basedregimens using new data sources, including aggregated data from ViiV Healthcareclinical trials, the Observational Pharmaco-Epidemiology Research & Analysis (OPERA®)25cohort, and cases spontaneously reported to ViiV Healthcare; and put these findings in thecontext of results for other anchor drugs."
Depression was reported fornumerically more patients treated with EFV/TDF/FTC inSINGLE (44/419 [11%]) than for other treatment groups/trials. The proportion of depressioncases considered drug related by reporting investigators was highest in both treatment groupsfor SINGLE compared with the other 4 trials; this was also true for anxiety cases in the EFV-treatedpatients. A small proportion of either anxiety or depression cases were consideredserious (Supplementary Table 1) or of grade 3 to 4 intensity. SINGLE was the only study inwhich patients withdrew due to anxiety (EFV, n=4) or depression (DTG, n=1; EFV, n=6;Table 1).
Suicidality, including suicidal ideation, attempted suicide, and self-injurious behavior,occurred in 1% of patients across trials/treatment groups (DTG, 20/1672; comparator, 20/1681),and was considered drug related in 2/20 of the DTG-treated patients and 5/20 of the
comparator-treated patients (Table 1). Two patients, 1 treated with DTG and 1 with RAL,completed suicide. One separate, nonfatal case of suicidality with DTG and 3 additional nonfatalcases with comparators led to treatment withdrawal. The majority of cases resolved or improvedacross all trials (Supplementary Table 1). Of the 20 reported cases of suicidality in DTG-treatedpatients, 19 (95%) had a psychiatric history at baseline consistent with an increased risk forsuicide, including previous history of suicidal ideation (n=2), suicide attempts (n=6),depression/bipolar disorder (n=8), or other psychiatric conditions not further specified (n=3). Theone DTG-treated patient with no previous psychiatric history who attempted suicide, did so onDay 899; the event was associated with emotional triggers and considered unrelated to studydrug. Of the 20 cases of suicidality in comparator-treated patients, 16 (80%) had a psychiatrichistory at baseline, with a similar pattern as observed for DTG, 2 had socioeconomic triggers,and 2 had no obvious underlying risk factors. The DTG-treated patient who completed suicidewas male, of unknown age, and had a previous medical history of bipolar disorder requiring treatment, suicidal ideation, and suicide attempt. The RAL-treated patient who completedsuicide was also male, aged 22 years, and had no reported underlying risk factors or triggers.Neither case of completed suicide was considered drug related."
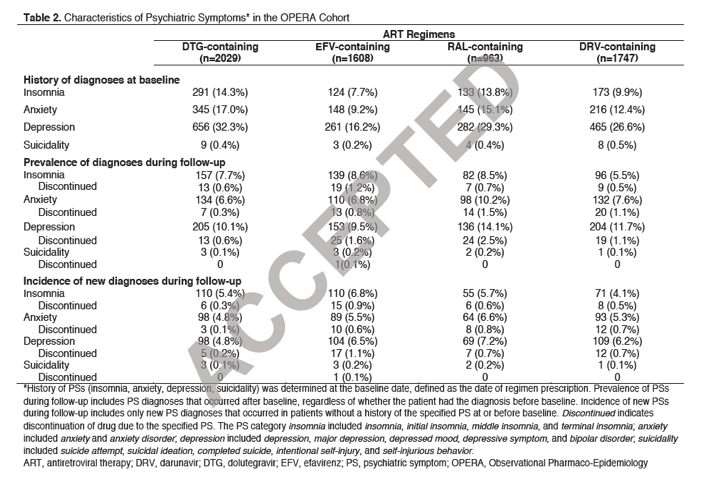
"History of anxiety, depression, or insomnia was highest in DTG-treated patients and lowest in EFV-treated patients.Despite this, the prevalence of anxiety, depression, and insomnia was similar across all 4 anchor drugs, except for a higher prevalence of anxiety and depression in RAL-treated patients and a lower prevalence of insomnia in DRV-treated patients (Table 2)......In patients without ahistory of these psychiatric conditions at baseline, the incidence of anxiety, depression, and insomnia was similar across all 4 anchor drugs. However, treatment discontinuations for these symptoms were lowest for DTG-treated patients (range 0.1% to 0.3% compared with a range of 0.5% to 1.1% for the other 3 anchor drugs)"
"The analysis of 3 different data sources shows that, similar to other frequently prescribed anchor drugs to treat HIV infection, PSs are also reported in DTG-treated patients. These events are reported with low frequency and most are generally mild to moderate in intensity, non-serious (with the exception of suicidality), and rarely necessitate DTG discontinuation
In those patients who developed depression whose psychiatric history was known (34/100), approximately 85% had a history of depression before drug initiation. An absence of past historyof depressive symptoms was only identified in 3 cases.
Among cases of suicidality with reported TTO (~50%), suicidality typically occurredwithin 1 month of starting treatment with DTG or ABC/DTG/3TC. In the 40% of suicidality casesfor which past psychiatric history was available, 16 of 20 patients had a psychiatric conditionbefore starting treatment. Five patients completed suicide (DTG, n=4; ABC/DTG/3TC, n=1).Four of the 5 cases occurred within 6 months of commencing treatment (Supplementary Table3). Three of these patients had a history of depression and 1 previously attempted suicide. Inthe case for which psychiatric history was not available, there was evidence suggesting drugabuse and psychiatric instability when the patient started treatment. The final case involved a55-year-old man living with HIV for approximately 30 years who had multiple co-morbidities; thesuicide was considered unrelated to DTG.
--------------------------
Clinical Trials
This is an analysis of phase III clinical trials that investigated DTG 50 mg once daily in adultsand had at least 48 weeks of data as of April 2016, which includes the SPRING-2, FLAMINGO,SINGLE, ARIA, and SAILING studies. Design details for each study have been previouslydescribed.26-30 Briefly, in all 5 studies, patients were randomized 1:1 to receive either DTG- orcomparator-containing treatment (RAL in SPRING-2 and SAILING, efavirenz [EFV]/tenofovirdisoproxil fumarate [TDF]/emtricitabine [FTC] in SINGLE, darunavir + ritonavir [DRV/r] inFLAMINGO, and atazanavir + ritonavir [ATV/r] in ARIA). In 4 of the studies, DTG wasadministered as the single active preparation; while in the ARIA study, the fixed-dosecombination tablet of abacavir (ABC)/DTG/lamivudine (3TC) was used. SPRING-2, SINGLE,and SAILING were all double-blind, double-dummy, controlled trials. Patients in SAILING hadbeen treated with ART (not including INSTIs), had documented resistance to 2 or moreantiretroviral drug classes, and had background therapy (BT) with 1 to 2 fully active agents.27
Patients in the other 4 trials were ART naive. For SPRING-2 and FLAMINGO, BT in each armwas ABC/3TC or TDF/FTC; BT for DTG-treated patients in SINGLE was ABC/3TC, and BT wasTDF/FTC for ATV/r-treated patients in ARIA. All patients treated with ABC-containing regimenswere negative for the HLA-B*5701 allele.26-30 For all 5 studies, ethics committee approval wasobtained at all participating centres in accordance with the principles of the 2008 Declaration ofHelsinki. Each patient provided written informed consent before undergoing study procedures.In all of these clinical trials, PSs were captured through AE and serious AE (SAE) reportingpost baseline at scheduled study visits.26-
OPERA Cohort
Using electronic medical record data from the OPERA database, 6347 HIV-positive patientswere identified who initiated DTG-, EFV-, RAL- or DRV-based regimens (commonly prescribedanchor drugs) regardless of prior ART treatment from January 1, 2013 (the first year DTG wasmarketed) through April 30, 2016.Patients were observed from the start of these regimens untilthe first of the following censoring events: discontinuation of the agent of interest, cessation ofcontinuous clinical activity, death, or study end. Patients exposed to any of the drugs of interestbefore the observation period were excluded. The endpoints included diagnoses consistent withanxiety, depression, insomnia, and suicidality identified from the medical record and event timeto onset (TTO) calculated from treatment initiation to first occurrence of a PS. Patientdemographics and clinical characteristics, including past history of psychiatric conditions, wereexamined.
|
|
| |
| |
|
|
|