| |
Inflammation, Immune Activation, Immunosenescence, and Hormonal Biomarkers in the Frailty-Related Phenotype of Men With or at Risk for HIV Infection - lower CD4, detectable viral load, more inflammation, lower testosterone, HIV associated with senescence & immune activation.....Exercise & Diet recommended
|
| |
| |
Download the PDF here
"few interventions outside of exercise have been shown to effectively reverse the trajectory of frailty.....interventions should focus on limiting ongoing exposures to inflammation through lifestyle factors, such as maintenance of a healthy weight and diet, physical activity, and adequate sleep; optimizing management of comorbid conditions; and preservation of gonadal function (ie, early initiation of ART and avoidance of alcohol, marijuana, and opiates). Ultimately, early and long-lasting interventions that affect multiple pathways will probably prove most effective in slowing or preventing frailty, particularly in older HIV-infected adults"
Frail Men in MACS - lower CD4, detectable viral load, more inflammation, lower testosterone, HIV associated with senescence & immune activation.....Exercise & Diet recommended
"consistent with the concept of multi system dysregulation: we have shown that [inflammation] IL-6 and hsCRP levels & lower DHEA-S and testosterone levels were associated with frailty among HIV-infected men.....HIV-infected men had significantly higher percentages of CD38+HLA-DR+ and CD28- T cells than HIV-uninfected men[markers of immune activation or senescence].... frail men had a similar proportion of low nadir CD4+ T-cell count but a higher proportion of current CD4+ T-cell count <350/μL and detectable HIV-1 viral load than HIV-infected non frail men"
Both frailty and HIV infection are associated with heightened inflammation, hormonal abnormalities, and impairments in the immune system [10, 11]. The extent to which these factors are driven by HIV infection or frailty, particularly among virologically suppressed HIV-infected individuals, is less clear. A better understanding of the underlying mechanisms that drive frailty can inform the most appropriate and efficient pathways to target for prevention and treatment of frailty. The goal of the current study was to describe differences in levels of inflammatory, immune, and hormonal markers by frailty status among persons with HIV infection (ie, comparing HIV-infected men with or without frailty) and by HIV serostatus without the influence of frailty (ie, comparing nonfrail HIV-infected and HIV-uninfected men). We hypothesized that both frailty and HIV infection would be associated with abnormalities across all 3 domains.
"......few interventions outside of exercise have been shown to effectively reverse the trajectory of frailty...."....."For now, interventions should focus on limiting ongoing exposures to inflammation through lifestyle factors, such as maintenance of a healthy weight and diet, physical activity, and adequate sleep; optimizing management of comorbid conditions; and preservation of gonadal function (ie, early initiation of ART and avoidance of alcohol, marijuana, and opiates). Ultimately, early and long-lasting interventions that affect multiple pathways will probably prove most effective in slowing or preventing frailty, particularly in older HIV-infected adults."
DISCUSSION: Prior studies suggest a causal role of inflammation in muscle mass decline, muscle turnover, and weight loss, contributing to components of frailty [44-47]. In the present study, associations persisted in multivariate models adjusted for comorbid conditions, suggesting that the frailty-associated inflammation and hormonal dysregulation were not merely a result of greater comorbid disease burden among frail participants. Thus, our findings emphasize the potential importance of inflammation (IL-6 and hsCRP) and hormonal dysregulation in frailty among HIV-infected adults. However, considering the cross-sectional design and the small but significant difference, our findings cannot provide a basis for recommending testosterone or DHEA-S replacement or anti-inflammatory therapy as a treatment for frailty
The degree to which multisystem regulation in older, HIV-infected men is altered by HIV infection versus the presence of frailty has not previously been described, to our knowledge. In the current study, by analyzing both HIV status and frailty status together, we have shown that IL-6 and hsCRP levels were associated with frailty among HIV-infected men. We have also shown for the first time that lower DHEA-S and testosterone levels were also associated with frailty in those men, consistent with the concept of multisystem dysregulation. In contrast, HIV serostatus but not frailty was associated with cellular immune activation and immunosenescence. Even with effective ART, inflammatory markers remain elevated among HIV-infected compared with HIV-uninfected controls [21]. Although pronounced differences by HIV serostatus were expected, the adjusted differences were driven more by frailty for IL-6 and hsCRP. Overall, the association between inflammation and frailty is consistent with findings in several prior studies in HIV-infected populations, irrespective of the frailty definition, age range, or HIV risk factors of the population studied.It is well recognized that markers of T-cell senescence and activation are higher among HIV-infected [27] than among HIV-uninfected persons, but the degree to which these elevations are attributable to frailty or to HIV is not clear. The lack of association of these cellular markers with frailty in our population differed from findings in a prior study [22], which found a strong association between frailty and immune activation (the percentage of CD38+HLA-DR+ CD8+ T cells) [22] ....Insulin resistance or diabetes and low DHEA-S and testosterone levels have been associated with frailty and components of frailty, including fatigue, weakness, and low energy, in multiple studies of HIV-uninfected cohorts [10, 28-33]. In HIV-infected populations, frailty (by varying definitions) has been associated with diabetes [34, 35], low testosterone levels [36], and low IGF-1 levels [37], each in separate cohorts. Although low levels of DHEA-S were seen in asymptomatic HIV disease before HAART [38] and have been associated with progression to AIDS [39], no prior studies in HIV-infected populations had shown an association with frailty. Whether treatments to replace or normalize these hormones might result in improvement in HIV-infected adults remains to be clearly established in either HIV-infected or HIV-uninfected populations. Data on the effects of DHEA supplementation on physical function in the general elderly population are inconclusive [40], with some studies [41], but not all [42], demonstrating improved strength and function. Furthermore, the benefit of testosterone replacement therapy on components of frailty remains controversial [43].[full results and discussion below]
⇒ Cellular markers of immune activation or senescence did not differ significantly by frailty status among HIV-infected men (Figure 2A). In contrast, HIV-infected men had significantly higher percentages of CD38+HLA-DR+ and CD28- T cells than HIV-uninfected men (Figure 2B).
⇒ frail HIV-infected men had a much higher prevalence of depressive symptoms by CES-D (54.8%) than nonfrail HIV-infected and HIV-uninfected men (5.0% and 4.7%, respectively, Table 1).
⇒ Among HIV-infected men, frail men had a similar proportion of low nadir CD4+ T-cell count but a higher proportion of current CD4+ T-cell count <350/μL and detectable HIV-1 viral load than HIV-infected nonfrail men.
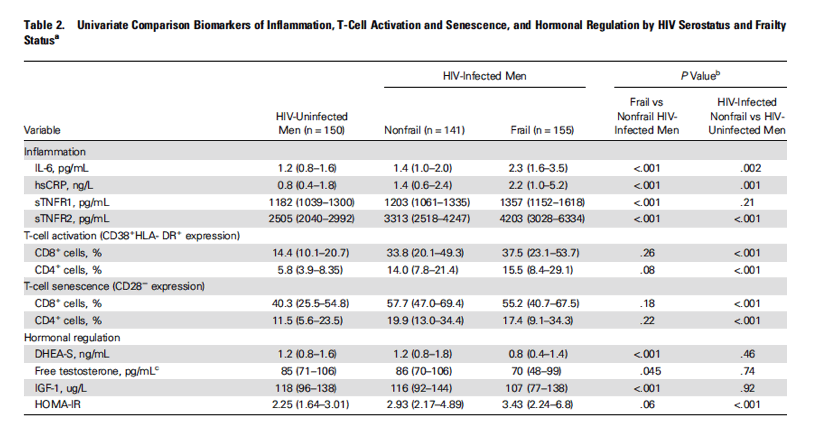
⇒ Levels of inflammatory markers (IL-6, hsCRP, sTNFR1, and sTNFR2) were highest among frail HIV-infected men and lowest among nonfrail HIV-uninfected men.
⇒ Markers of immune activation (percentage of CD38+HLA-DR+ CD4+ or CD8+ T cells) and senescence (percentage of CD28- CD4+ or CD8+ T cells) were similar among HIV-infected frail and HIV-infected nonfrail men and significantly lower among HIV-uninfected men (all P < .001).
⇒ Frail HIV-infected men had significantly lower levels of DHEA-S (P < .001), free testosterone (P = .045), and IGF-1 (P < .001) and a trend toward worsening insulin resistance (HOMA-IR; P = .06) compared with nonfrail, HIV-infected men. In contrast, only insulin resistance was worse by HIV status, with significantly greater HOMA-IR (P < .001) among the nonfrail HIV-infected than among the nonfrail HIV-uninfected men.
⇒ Among HIV-infected men, frailty was associated with 52% higher IL-6 (P < .001) and 69% higher hsCRP concentrations (P < .001; Figure 1A). Adjusted differences between the groups in markers of inflammation, immune activation and senescence, and hormone biomarkers are shown in Figures 1, 2, and 3, respectively.
------------------
Inflammation, Immune Activation, Immunosenescence, and Hormonal Biomarkers in the Frailty-Related Phenotype of Men With or at Risk for HIV Infection
JID Jan 15 2017
Kristine M. Erlandson,1 Derek K. Ng,2 Lisa P. Jacobson,2 Joseph B. Margolick,2 Adrian S. Dobs,2 Frank J. Palella Jr,4 Jordan E. Lake,5 Hanhvy Bui,2
Lawrence Kingsley,6 and Todd T. Brown3
1University of Colorado School of Medicine, Aurora; 2Johns Hopkins Bloomberg School of Public Health, and 3Johns Hopkins School of Medicine, Baltimore, Maryland; 4Northwestern University Feinberg School of Medicine, Chicago, Illinois; 5UTHealth McGovern School of Medicine Houston, Texas; 6University of Pittsburgh, Pennsylvania
Background
The extent to which inflammation, immune activation/immunosenescence, and hormonal abnormalities are driven by human immunodeficiency virus (HIV) or frailty is not clear.
Methods
HIV-infected frail men (n = 155) were matched to nonfrail, HIV-infected (n = 141) and HIV-uninfected (n = 150) men by age, calendar year, and antiretroviral therapy use (HIV-infected men only). Frailty was defined by ≥3 frailty-related phenotype criteria (weight loss, exhaustion, low activity, slowness) at ≥2 visits, or at 1 visit with ≥1 criteria at ≥2 visits. The following measurements were obtained: interleukin 6, high-sensitivity C-reactive protein, soluble receptors for tumor necrosis factor α 1 and 2, the percentages of CD4+CD28-, CD8+CD28-, CD4+CD38+HLA-DR+, and CD8+CD38+HLA-DR+ T cells, dehydroepiandrosterone sulfate, free testosterone, homeostatic model assessment of insulin resistance, and insulin-like growth factor 1. Log-linear regressions were adjusted for a priori selected covariates to determine differences by frailty and HIV status.
Results
In multivariate analyses adjusted for covariates, frailty was associated among HIV-infected men with higher interleukin 6 and high-sensitivity C-reactive protein and lower free testosterone and dehydroepiandrosterone levels. In contrast, HIV infection but not frailty was associated with significantly greater immune senescence (percentage of CD4+CD28- or CD8+CD28- T cells) and immune activation (percentages of CD4+CD38+HLA-DR+ and CD8+CD38+HLA-DR+ T cells).
Conclusions
Frailty among HIV-infected men was associated with increased inflammation and lower hormone levels, independent of comorbid conditions. Interventions targeting these pathways should be evaluated to determine the impact on prevention or reversal of frailty among HIV-infected men.
Frailty is a syndrome characterized by an increased vulnerability to stressors in the face of limited physiologic reserve [1]. A frailty phenotype was defined by Fried et al [2] as impairment in ≥3 of 5 domains: physical slowness, fatigue, low activity, weakness, and physical shrinking. Other authors have used a model of an accumulation of deficits to define frailty, or have modified the original phenotype described by Fried et al, using a combination of objective and subjective measures of health to fit the population or available data [3]. Regardless of the specific definition, frailty has been associated with increased healthcare costs and utilization, poor outcomes after surgical procedures, increased need for skilled nursing care among other outcomes, and ultimately, an increased risk of death [4].
A similarity between the syndrome of frailty and AIDS was recognized early in the human immunodeficiency virus (HIV) era [5]. The occurrence of a frailty-related phenotype was later reported in the Multicenter AIDS Cohort Study (MACS), wherein the highest proportion of the frailty-related phenotype was among persons with low CD4+ T-cell counts or those not receiving antiretroviral therapy (ART) [6, 7]. A subsequent MACS study demonstrated that the frailty-related phenotype was associated with lower AIDS-free and overall survival, even among persons who achieved HIV-1 virologic suppression [8]. A frailty index developed from self-reported data in the Veterans Aging Cohort Study (VACS) was likewise predictive of hospitalization and mortality rates [9]. These phenotypes are different than the original frailty definition offered by Fried et al [2] in that they include subjective measures only and lack extensive validation; however, their association with mortality suggests that both are valuable measures of vulnerability to stressors.
Both frailty and HIV infection are associated with heightened inflammation, hormonal abnormalities, and impairments in the immune system [10, 11]. The extent to which these factors are driven by HIV infection or frailty, particularly among virologically suppressed HIV-infected individuals, is less clear. A better understanding of the underlying mechanisms that drive frailty can inform the most appropriate and efficient pathways to target for prevention and treatment of frailty. The goal of the current study was to describe differences in levels of inflammatory, immune, and hormonal markers by frailty status among persons with HIV infection (ie, comparing HIV-infected men with or without frailty) and by HIV serostatus without the influence of frailty (ie, comparing nonfrail HIV-infected and HIV-uninfected men). We hypothesized that both frailty and HIV infection would be associated with abnormalities across all 3 domains.
Study Population
The MACS is a prospective study of the natural and treated history of HIV infection among men who have sex with men in the United States, with sites in Baltimore, Maryland-Washington, DC; Chicago, Illinois; Pittsburgh, Pennsylvania; and Los Angeles, California. Enrollment of 6972 participants occurred during 3 time periods: 1984-1985, 1987-1990, and 2001-2003. At each semiannual study visit, physical examinations were performed, questionnaires administered, and blood samples collected for laboratory testing and storage. Self-reported use of antiretroviral medications was summarized at each visit to define prior and current use of ART. Informed consent was obtained from each participant, and approval was provided by each local institutional review board.
Selection of Cases and Controls, by Exposure Status
This analysis employed a matched study design of HIV-infected men without AIDS (defined as no history of an AIDS-defining illness [12]) and HIV-uninfected men in the MACS. The exposure of interest was frailty, defined as follows. First, the frailty-related phenotype was summarized by 4 self-reported subjective measures [6] describing weight loss, exhaustion, low activity levels, and slowness. Weight loss was defined as self-reported unintentional weight loss of ≥10 lb (4.54 kg) since the previous visit. Other criteria were derived from the Short-Form (SF)-36: exhaustion was defined as answering yes to the question: "During the past 4 weeks, as a result of your physical health, have you had difficulty performing your work or other activities (for example, it took extra effort)?" Low activity was defined as feeling "limited a lot" in response to: "Does your health now limit you in vigorous activities, such as running, lifting heavy objects, participating in strenuous sports?" Slowness was defined as feeling "limited a lot" in response to: "Does your health now limit you in walking several blocks?" HIV-infected men were considered frail if they had either (1) ≥2 visits meeting ≥3 frailty-related phenotype criteria or (2) 1 visit meeting ≥3 criteria and 2 subsequent visits meeting 1-2 criteria. The index visit (ie, study entry) for each case was the first visit at which frailty was identified. HIV-infected and HIV-uninfected controls with no history of meeting any frailty-related phenotype criteria were matched to each case patient by age within 3 years and calendar year of visit. Among HIV-infected participants, controls were also matched at the index visit by the reported use of HIV therapy, categorized as highly active ART (HAART), non-HAART therapy, or no ART. The definition of HAART was guided by the Department of Health and Human Services guidelines [13].
RESULTS
Of 4005 potentially eligible participants seen between 1994 and 2010, a total of 155 HIV-infected men met our frailty criteria: 101 (65%) had ≥2 visits with ≥3 frailty-related phenotype criteria; 54 (35%) had 1 visit with ≥3 frailty-related phenotype criteria and ≥2 visits with 1-2 criteria. HIV-infected frail men were matched to 141 nonfrail HIV-infected and 150 nonfrail HIV-uninfected men. The median ages were between 47 and 49 years, and the median year of the index visits was 2006 (Table 1).
Characteristics of the study population are detailed in Table 1. Groups were significantly different in many characteristics; for example, frail HIV-infected men had a much higher prevalence of depressive symptoms by CES-D (54.8%) than nonfrail HIV-infected and HIV-uninfected men (5.0% and 4.7%, respectively, Table 1). Among HIV-infected men, frail men had a similar proportion of low nadir CD4+ T-cell count but a higher proportion of current CD4+ T-cell count <350/μL and detectable HIV-1 viral load than HIV-infected nonfrail men. Similar proportions of frail and nonfrail HIV-infected participants were receiving HAART (74.8% vs 72.3%, respectively). Frail men were more likely to report testosterone therapy use (13.8% vs 5.2%), although 16% (n = 25) and 18% (n = 26) of frail and nonfrail HIV-infected were missing data on self-reported testosterone use ( not collected for HIV-uninfected men).
Differences in markers of inflammation, immune activation and senescence, and hormonal dysfunction between HIV-uninfected men, HIV-infected nonfrail, and HIV-infected frail men are shown in Table 2 (unadjusted). Levels of inflammatory markers (IL-6, hsCRP, sTNFR1, and sTNFR2) were highest among frail HIV-infected men and lowest among nonfrail HIV-uninfected men. Markers of immune activation (percentage of CD38+HLA-DR+ CD4+ or CD8+ T cells) and senescence (percentage of CD28- CD4+ or CD8+ T cells) were similar among HIV-infected frail and HIV-infected nonfrail men and significantly lower among HIV-uninfected men (all P < .001). Frail HIV-infected men had significantly lower levels of DHEA-S (P < .001), free testosterone (P = .045), and IGF-1 (P < .001) and a trend toward worsening insulin resistance (HOMA-IR; P = .06) compared with nonfrail, HIV-infected men. In contrast, only insulin resistance was worse by HIV status, with significantly greater HOMA-IR (P < .001) among the nonfrail HIV-infected than among the nonfrail HIV-uninfected men.
Adjusted differences between the groups in markers of inflammation, immune activation and senescence, and hormone biomarkers are shown in Figures 1, 2, and 3, respectively. Among HIV-infected men, frailty was associated with 52% higher IL-6 (P < .001) and 69% higher hsCRP concentrations (P < .001; Figure 1A). Only sTNFR2 levels were significantly higher among HIV-infected versus HIV-uninfected nonfrail men (22% higher; P < .001; Figure 1B). Cellular markers of immune activation or senescence did not differ significantly by frailty status among HIV-infected men (Figure 2A). In contrast, HIV-infected men had significantly higher percentages of CD38+HLA-DR+ and CD28- T cells than HIV-uninfected men (Figure 2B). Among HIV-infected men, the presence of frailty was significantly associated with lower free testosterone (17% lower; P = .02) and lower DHEA-S (18% lower; P = .04), with a trend toward worsened insulin resistance (20% higher HOMA-IR; P = .051; Figure 3A). In contrast, among nonfrail men, HIV infection was significantly associated with greater insulin resistance (26% higher HOMA-IR; P = .003) but not with DHEA-S, free testosterone, or IGF-1 levels (Figure 3B).
To further investigate the potential impact of detectable compared with undetectable HIV-1 RNA, a sensitivity analysis was performed, restricting the analysis to HIV-uninfected men, and HIV-infected frail (n = 76) and nonfrail (n = 86) men with an undetectable viral load (
Supplemental Table 1
). Univariate comparisons were similar to Table 2 and did not change significance level, except that the percentage of CD4+ T cells with CD28- expression was significantly lower among frail versus nonfrail HIV-infected men. In the adjusted comparison (
Supplemental Table 2
), similar to the overall findings (Figures 1A-C), the HIV-infected frail men had higher hsCRP (P = .005), lower free testosterone (P = .01) and greater insulin resistance (P = .01) than HIV-infected nonfrail men. The difference in IL-6 was attenuated and no longer significant (P = .15), and frail HIV-infected men had a significantly lower percentage of CD4+CD28- T cells (P = .02). Among HIV-infected vs HIV-uninfected men, the difference in sTNFR2 was attenuated but remained significant (P = .049); differences in markers of immune senescence persisted (all P < .001).
DISCUSSION
The degree to which multisystem regulation in older, HIV-infected men is altered by HIV infection versus the presence of frailty has not previously been described, to our knowledge. In the current study, by analyzing both HIV status and frailty status together, we have shown that IL-6 and hsCRP levels were associated with frailty among HIV-infected men. We have also shown for the first time that lower DHEA-S and testosterone levels were also associated with frailty in those men, consistent with the concept of multisystem dysregulation. In contrast, HIV serostatus but not frailty was associated with cellular immune activation and immunosenescence.
Even with effective ART, inflammatory markers remain elevated among HIV-infected compared with HIV-uninfected controls [21]. Although pronounced differences by HIV serostatus were expected, the adjusted differences were driven more by frailty for IL-6 and hsCRP. Overall, the association between inflammation and frailty is consistent with findings in several prior studies in HIV-infected populations, irrespective of the frailty definition, age range, or HIV risk factors of the population studied. Erlandson et al [22] found significantly higher levels of IL-6 but not CRP among frail versus nonfrail HIV-infected adults, perhaps owing to differences in liver function or other downstream inflammatory signaling between the populations. In a separate analysis of men in the MACS [23], HIV-infected men who were frail according to the criteria of Fried et al [2] had 50% higher CRP concentrations than nonfrail HIV-infected men. In the AIDS linked to the intraVenous experience cohort of HIV-infected and HIV-uninfected injection drug users, both IL-6 and sTNFR1 levels increased with increasing frailty, and this relationship was slightly stronger among HIV-infected participants [24]. In contrast, in the VACS, inflammation was associated with a higher score on the VACS Index, an index of HIV-related variables and other comorbid conditions, but not with a subjective frailty index [25]. In addition, in the AGEhiv Cohort, frailty was not associated with markers of inflammation or monocyte activation [26]. Differences in HIV disease severity, frailty definitions, comorbid conditions, coinfections, and other unrecognized confounders may account for discrepancies between studies, but as a whole these studies add to the growing literature on the relationship between frailty and the chronic inflammation of HIV infection.
It is well recognized that markers of T-cell senescence and activation are higher among HIV-infected [27] than among HIV-uninfected persons, but the degree to which these elevations are attributable to frailty or to HIV is not clear. The lack of association of these cellular markers with frailty in our population differed from findings in a prior study [22], which found a strong association between frailty and immune activation (the percentage of CD38+HLA-DR+ CD8+ T cells) [22]. In the prior study, the median percentage of CD38+HLA-DR+ CD8+ T cells was 15%, all participants were receiving ART, and 96% were virologically suppressed [22]. In contrast, in the current study the CD38+HLA-DR+ CD8+ T cells were much higher for HIV-infected men, even when restricted to those with virologic suppression (
Supplemental Tables
). The reasons for these differences are unclear, but they could be partially explained by different characterizations of frailty.
Insulin resistance or diabetes and low DHEA-S and testosterone levels have been associated with frailty and components of frailty, including fatigue, weakness, and low energy, in multiple studies of HIV-uninfected cohorts [10, 28-33]. In HIV-infected populations, frailty (by varying definitions) has been associated with diabetes [34, 35], low testosterone levels [36], and low IGF-1 levels [37], each in separate cohorts. Although low levels of DHEA-S were seen in asymptomatic HIV disease before HAART [38] and have been associated with progression to AIDS [39], no prior studies in HIV-infected populations had shown an association with frailty. Whether treatments to replace or normalize these hormones might result in improvement in HIV-infected adults remains to be clearly established in either HIV-infected or HIV-uninfected populations. Data on the effects of DHEA supplementation on physical function in the general elderly population are inconclusive [40], with some studies [41], but not all [42], demonstrating improved strength and function. Furthermore, the benefit of testosterone replacement therapy on components of frailty remains controversial [43].
Several limitations of our study should be mentioned. First, we used the frailty-related phenotype to establish a classification of frailty. This phenotype has been used in several prior studies within the MACS Cohort, because grip strength and gait speed were not added to the MACS until 2007. The phenotype is associated with increased mortality rates and poor outcomes [8], but it relies on subjective report and therefore may reflect a greater predominance of depressive symptoms, subjective fatigue, and self-image than the frailty phenotype described by Fried et al [8]. Second, the median year of assessment was 2006, so some of our data preceded the HAART era and were subject to a lack of viral suppression. Furthermore, relatively few observations were from the modern ART era of the potent integrase-strand transfer inhibitors. Matching by calendar year and ART use, with further adjustment for virologic suppression, minimized the ART differences while maximizing the available data across diverse HIV treatment periods. The MACS includes only men who have sex with men, and the present study included predominantly middle-aged white men; thus, generalizability to women, much older populations, or populations with greater racial/ethnic diversity or differing HIV risk factors may be limited. Furthermore, no HIV-uninfected frail control group was included. Differences in body fat and muscle mass beyond BMI may have influenced markers of inflammation, activation, and hormone dysfunction, but image-based body composition measures were not available for most participants. Similarly, additional measures of immune senescence, such as CD57 or proliferation assays, may have provided a better assessment of the true senescence status. Finally, the cross-sectional nature of the data does not allow inferences to be made regarding causality.
Prior studies suggest a causal role of inflammation in muscle mass decline, muscle turnover, and weight loss, contributing to components of frailty [44-47]. In the present study, associations persisted in multivariate models adjusted for comorbid conditions, suggesting that the frailty-associated inflammation and hormonal dysregulation were not merely a result of greater comorbid disease burden among frail participants. Thus, our findings emphasize the potential importance of inflammation (IL-6 and hsCRP) and hormonal dysregulation in frailty among HIV-infected adults. However, considering the cross-sectional design and the small but significant difference, our findings cannot provide a basis for recommending testosterone or DHEA-S replacement or anti-inflammatory therapy as a treatment for frailty. Indeed, few interventions outside of exercise have been shown to effectively reverse the trajectory of frailty [48]. Results from ongoing studies on the anti-inflammatory roles of statins, angiotensin-converting enzyme inhibitors/angiotensin-receptor blockade, metformin, or probiotics may support a role for these therapies in the future [49]. For now, interventions should focus on limiting ongoing exposures to inflammation through lifestyle factors, such as maintenance of a healthy weight and diet, physical activity, and adequate sleep; optimizing management of comorbid conditions; and preservation of gonadal function (ie, early initiation of ART and avoidance of alcohol, marijuana, and opiates). Ultimately, early and long-lasting interventions that affect multiple pathways will probably prove most effective in slowing or preventing frailty, particularly in older HIV-infected adults.
|
|
| |
| |
|
|
|