| |
Aerobic Interval Training Versus Continuous Moderate Exercise as a Treatment for the Metabolic Syndrome
|
| |
| |
Download the PDF here
"The present study demonstrates that high-intensity exercise training is superior to moderate-intensity training in reversing risk factors of the metabolic syndrome.....We demonstrated here that high-intensity exercise increased V̇o2max to a higher degree than moderate-intensity exercise in patients with the metabolic syndrome....AIT caused a significant improvement in fasting blood glucose compared with the other groups......High-intensity training [AIT] improved endothelial function (FMD) more than continuous [moderate] exercise......CME and AIT reduced systolic and diastolic blood pressures by ≈ 10 and 6 mm Hg, respectively. On the basis of a meta-analysis of 1 million adults, a blood pressure lowering of this magnitude would, in the long term, be associated with ≈ 40% and 30% decreases in the risks of premature deaths resulting from stroke and ischemic heart disease.....Physical activity and a healthy lifestyle promote insulin sensitivity, whereas a sedentary lifestyle and a Western diet are associated with insulin resistance. In our study, AIT promoted insulin sensitivity and β-cell function more than CME. Increased insulin sensitivity and β-cell function after exercise have been proposed to result from peripherally enhanced insulin response and signaling.30 Consistently, exercise, particularly high-intensity training, improved IR phosphorylation (and activation) by insulin in muscle and fat tissue. The mechanism for improved insulin action in muscle by AIT is not clear...... AIT reduced more of the other factors of the metabolic syndrome, including insulin sensitivity (HOMA) (group difference, P<0.05; Tables 2 and 3⇑ ) compared with CME. At the end of the training period, 46% (P<0.05) in the AIT and 37% in the CME groups (P=0.23; between-group difference, P<0.05) were no longer diagnosed with the metabolic syndrome; in the control group, the syndrome persisted in all subjects (Table 3)."
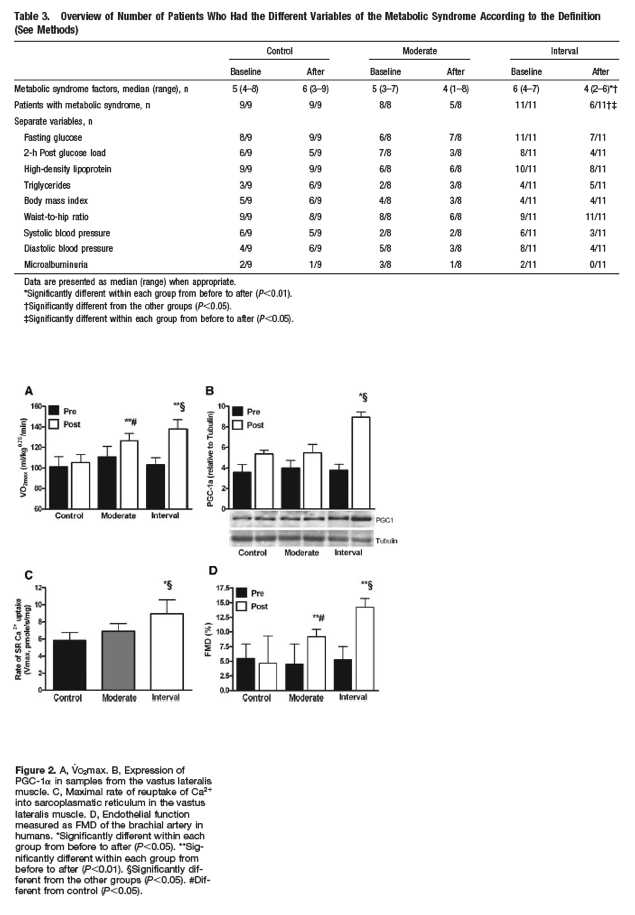
Aerobic Capacity - AIT and CME increased V̇o2max by 35% and 16% (P<0.01), respectively (group difference, P<0.01; Figure 2A). Only AIT had a significantly increased peak O2 pulse (P<0.05; between-group difference, P<0.001; Table 2).
------------------
Aerobic Interval Training Versus Continuous Moderate Exercise as a Treatment for the Metabolic Syndrome
A Pilot Study
Circulation 2008 - Arnt Erik Tjonna, Sang Jun Lee, oivind Rognmo, Tomas O. Stolen, Anja Bye, Per Magnus Haram, Jan Pål Loennechen, Qusai Y. Al-Share, Eirik Skogvoll, Stig A. Slordahl, Ole J. Kemi, Sonia M. Najjar, Ulrik Wisloff
Abstract
Background- Individuals with the metabolic syndrome are 3 times more likely to die of heart disease than healthy counterparts. Exercise training reduces several of the symptoms of the syndrome, but the exercise intensity that yields the maximal beneficial adaptations is in dispute. We compared moderate and high exercise intensity with regard to variables associated with cardiovascular function and prognosis in patients with the metabolic syndrome.
Methods and Results- Thirty-two metabolic syndrome patients (age, 52.3±3.7 years; maximal oxygen uptake [V o2max], 34 mL ⋅ kg-1 ⋅ min-1) were randomized to equal volumes of either moderate continuous moderate exercise (CME; 70% of highest measured heart rate [Hfmax]) or aerobic interval training (AIT; 90% of Hfmax) 3 times a week for 16 weeks or to a control group. V̇2max increased more after AIT than CME (35% versus 16%; P<0.01) and was associated with removal of more risk factors that constitute the metabolic syndrome (number of factors: AIT, 5.9 before versus 4.0 after; P<0.01; CME, 5.7 before versus 5.0 after; group difference, P<0.05). AIT was superior to CME in enhancing endothelial function (9% versus 5%; P<0.001), insulin signaling in fat and skeletal muscle, skeletal muscle biogenesis, and excitation-contraction coupling and in reducing blood glucose and lipogenesis in adipose tissue. The 2 exercise programs were equally effective at lowering mean arterial blood pressure and reducing body weight (-2.3 and -3.6 kg in AIT and CME, respectively) and fat.
Endothelial Function
AIT and CME improved FMD by 9% (P<0.001) and 5% (P<0.001), respectively (group difference, P<0.001; Figure 2D). Endothelium-independent dilatation by the administration of nitroglycerine sublingually was equal in all groups (data not shown).
We observed increased availability of NO (36±3%; P<0.03) after AIT but not CME (P=0.37; group difference, P<0.05). Furthermore, oxidized LDL, which negatively regulates the bioavailability of NO, was reduced by 17% (102±8 U/mL before versus 85±7 U/mL after; P<0.001) in AIT but not CME (not shown) (group difference, P<0.01).
Insulin Action in Skeletal Muscle and Fat Tissue
Because IR phosphorylation in skeletal muscle is an excellent surrogate measure of peripheral insulin sensitivity in obese and type 2 diabetic humans,17,18 phosphorylation of the IR β subunit in the presence of insulin was examined.Insulin induced a substantial increase (2.5- to 15-fold; P<0.001; Figure 3A) in receptor phosphorylation (and thus activation) in the vastus lateralis muscle after AIT compared with the other groups (P<0.03). In fat tissue, AIT induced a larger increase in receptor phosphorylation compared with the other groups (P<0.01; Figure 3B).
Aerobic Capacity - Of all established risk factors, low aerobic exercise capacity appears to be the strongest predictor of mortality.5 We demonstrated here that high-intensity exercise increased V̇o2max to a higher degree than moderate-intensity exercise in patients with the metabolic syndrome. Central O2 delivery and peripheral O2 use, in addition to skeletal muscle capacity, contribute to training-induced changes in V̇o2max.13 Accordingly, AIT improved maximal stroke volume (measured as peak O2 pulse) and Ca2+ cycling and mitochondrial capacity in skeletal muscle (as assessed by improved sarcoplasmic reticulum Ca2+ ATPase capacity and PGC-1α levels in the vastus lateralis muscle) to a larger extent than CME.
Conclusions- Exercise intensity was an important factor for improving aerobic capacity and reversing the risk factors of the metabolic syndrome. These findings may have important implications for exercise training in rehabilitation programs and future studies.
The metabolic syndrome is a cluster of cardiovascular risk factors, including elevated blood pressure, dyslipidemia, impaired glycemic control, and abdominal obesity.1 This syndrome affects ≈ 25% of the adult US population and is reaching an epidemic spread in parallel to obesity, which is estimated to affect ≈ 312 million people worldwide. With at least 1.1 billion overweight people, the incidence of the metabolic syndrome is expected to continue to rise,2 warranting thorough mechanistic understanding to enable optimal treatment at the socioeconomic scale.
"Clinical Perspective p 354"
The metabolic syndrome is associated with increased cardiovascular morbidity and mortality because individuals with the metabolic syndrome are 3 times more likely to die of coronary heart disease than healthy counterparts after adjustment for conventional cardiovascular risk factors.3 Aerobic fitness and endothelial function are reduced in individuals with the metabolic syndrome and have been proposed to be independent and strong predictors of mortality relative to other established risk factors.4,5 Although a direct cause-effect relationship has not been established, impaired aerobic capacity is a common factor underlying cardiovascular and metabolic diseases. Indeed, it has been well established that exercise training at least partly reverses the metabolic syndrome,3 but the optimal level of exercise needed to prevent and treat the metabolic syndrome and its associated cardiovascular abnormalities remains undefined.
Here, we address this issue by testing the efficacy of 2 distinctly different modes of exercise to reverse features of the metabolic syndrome. That is, adaptations were measured in response to either continuous moderate exercise (CME) or aerobic interval training (AIT) at a high intensity in humans with clinically defined metabolic syndrome.
Discussion
The present study emphasizes that exercise intensity was an important factor for improving aerobic capacity and reversing the risk factors of the metabolic syndrome, including endothelial function, insulin action, and lipogenesis, in individuals with the metabolic syndrome.
Aerobic Capacity
Of all established risk factors, low aerobic exercise capacity appears to be the strongest predictor of mortality.5 We demonstrated here that high-intensity exercise increased V̇o2max to a higher degree than moderate-intensity exercise in patients with the metabolic syndrome. Central O2 delivery and peripheral O2 use, in addition to skeletal muscle capacity, contribute to training-induced changes in V̇o2max.13 Accordingly, AIT improved maximal stroke volume (measured as peak O2 pulse) and Ca2+ cycling and mitochondrial capacity in skeletal muscle (as assessed by improved sarcoplasmic reticulum Ca2+ ATPase capacity and PGC-1α levels in the vastus lateralis muscle) to a larger extent than CME.
Similar weight loss between exercise intensities and a larger total reduction of the cardiovascular risk factors that constitute the metabolic syndrome after AIT are in line with epidemiological observations suggesting that it may be more important to become fit than to lose weight per se.19,20 Moreover, although obesity and aerobic capacity are strong and independent prognostics markers for cardiovascular mortality, the link between aerobic capacity and mortality seems to be stronger.19,20 This suggests that it is more beneficial to target aerobic capacity before weight loss, although improvement in both risk factors probably would be ideal. However, larger prospective studies are needed to address this issue.
The main goal of the present study was to evaluate the effect of exercise intensity on the metabolic syndrome by subjecting patients to 2 different exercise regimens that differ solely by their level of intensity. The high-intensity exercise was set to 90% of Hfmax and performed as aerobic interval exercise because this training method has previously yielded the greatest improvement in aerobic capacity over a relatively short time in healthy individuals21 and in patients with coronary artery disease,8 intermittent claudication,22 and postinfarction heart failure.13 The rationale behind interval training is that most evidence suggests that it is the pumping capacity of the heart (ie, stroke volume) that limits V̇o2max, and the interval design allows rest periods that enable the patients to complete short work periods at higher intensities, which thereby challenge the pumping ability of the heart more than what would be possible by lower intensities.
General agreement exists that we need better and more affordable prevention and treatment strategies to improve wide-scale health outcome and to slow the current epidemic of overweight to prevent the epidemic of metabolic syndrome from reaching global proportions and straining public health and the economy.23 Furthermore, weight regulation has a complex nature, so the likelihood of a pharmacological solution is small.24 In fact, no available drug is ideal at the present time for most patients with metabolic syndrome.25 The present study suggests that exercise in general and AIT in particular are partly or fully able to reverse the metabolic syndrome, suggesting that this may be a promising treatment strategy. However, despite the results presented here, the interval protocol may not be readily acceptable to the general population of patients with the metabolic syndrome. On the other hand, an exercise intensity of 90% of Hfmax corresponds to ≈ 20 bpm below maximum. This implies that the patients would have tolerated higher exercise intensities (but with shorter durations) and that most patients walk "uphill" on the treadmill to avoid running and thereby reduce the risk of musculoskeletal injuries caused by the mechanical load at high running speeds. Interestingly, informal comments from the patients in the exercise groups indicate that AIT patients found it motivating to have a varied procedure to follow during each training session, whereas those in the CME group found it "quite boring" to walk continuously during the whole exercise period. To extend the clinical usefulness of the exercise training to those not having access to heart rate monitors, we recently asked patients with postinfarction heart failure to indicate the level of effort on a Borg scale after each interval or during continuous walking, ie, training protocols identical to those in the present study, and found that Borg scores of 17±1 and 12±1 to correspond to AIT and CME, respectively.13 Unpublished data from our laboratory confirm these numbers in both healthy and overweight individuals. To obtain the intended exercise intensity during home-based interval training, we normally instruct patients to exercise so that they are breathing heavily and talking becomes uncomfortable during each interval without developing severe leg stiffness, and it is an absolute requirement that they should be able to perform 4-minute intervals in succession. These instructions were satisfactory in old patients with postinfarction heart failure13 and may therefore also be feasible for patients with the metabolic syndrome. These observations suggest that it may be possible to instruct patients to perform such training without monitoring heart rate.
Endothelial Function
High-intensity training improved endothelial function (FMD) more than continuous exercise. In keeping with the notion that exercise-induced improvement in vessel relaxation is mainly mediated by NO,26 we observed no group differences in endothelium-independent relaxation in the brachial artery after administrating nitroglycerine sublingually. Recently, Hambrecht et al27 demonstrated 2-fold-higher endothelial NO synthase and 4-fold higher endothelial NO synthase Ser1177 phosphorylation levels in the left internal mammary artery after 4 weeks of exercise training in patients with stable coronary artery disease compared with sedentary counterparts. This suggests that the effect of AIT is mediated by improved NO bioavailability. Consistent with this hypothesis, AIT but not CME normalized the level of blood glucose, insulin sensitivity, and the amount of oxidized LDL, which directly influence NO bioavailability.28 The reason for the differences in FMD between groups is not fully understood, but it seems reasonable to suggest that the low- and high-intensity training exercise programs affect shear stress on the walls of blood vessels differently during exercise training and that this yields differences in molecular responses.
Blood Pressure
CME and AIT reduced systolic and diastolic blood pressures by ≈ 10 and 6 mm Hg, respectively. On the basis of a meta-analysis of 1 million adults, a blood pressure lowering of this magnitude would, in the long term, be associated with ≈ 40% and 30% decreases in the risks of premature deaths resulting from stroke and ischemic heart disease.29
Insulin Action
Physical activity and a healthy lifestyle promote insulin sensitivity, whereas a sedentary lifestyle and a Western diet are associated with insulin resistance. In our study, AIT promoted insulin sensitivity and β-cell function more than CME. Increased insulin sensitivity and β-cell function after exercise have been proposed to result from peripherally enhanced insulin response and signaling.30 Consistently, exercise, particularly high-intensity training, improved IR phosphorylation (and activation) by insulin in muscle and fat tissue. The mechanism for improved insulin action in muscle by AIT is not clear. However, exercise is generally known to promote insulin action in muscle by decreasing the intracellular accumulation of triglycerides and promoting fatty acid oxidation.31 Consistently, AIT increased mitochondrial biogenesis in skeletal muscle of patients with the metabolic syndrome. In agreement with recent reports,32 AIT did not alter fatty acid transport into muscle, as indicated by intact FATP-1 levels. In contrast, the larger improvement in IR activation by high-intensity training in fat tissue was associated with a stronger reduction in fatty acid uptake and lipogenesis in this tissue, as suggested by reduced FATP-1 and FAS levels, respectively. Although the effect of exercise on visceral adiposity per se was not measured, decreased lipogenesis, together with reduced waist circumference and body weight and in parallel with increased circulating adiponectin levels in exercise, suggests decreased intraabdominal visceral obesity. Taken together, the data suggest that both exercise programs decreased body weight but that AIT was particularly beneficial for decreasing fatty acid transport into the adipose tissue and promoting the suppressive effect of insulin on lipogenesis in this tissue. Together with a reduction in plasma oxidized LDL levels, decreased lipogenesis in fat tissue by AIT demonstrates larger improvement in the overall fat metabolism in patients with the metabolic syndrome by high-intensity training.
The positive effect of AIT on fasting glucose levels and insulin sensitivity may explain some of the group differences in these parameters. The slight worsening in CME with respect to these variables is not fully understood, especially because no diet alterations occurred during the 16-week intervention period. However, this suggests that CME, in contrast to AIT, did not produce enough stimulus to revert the phenotype.
Study Limitations
The number of patients in our study was small, and information on the safety and injury risk of this training protocol in the general population is not known. However, in a number of studies in our laboratory,8,13,21,22 we have observed only 2 knee injuries in extremely overweight patients. These data are provocative but should encourage larger multicenter studies.
Conclusions
The present study demonstrates that high-intensity exercise training is superior to moderate-intensity training in reversing risk factors of the metabolic syndrome. The closely supervised training regimens and the comparable training volumes between the 2 exercise groups are strong features of our study and demonstrate the importance of exercise training in reducing the risks of metabolic syndrome. Exercise training, especially high intensity, appears to be highly beneficial in preventing the metabolic syndrome relative to any other currently known interventions. These findings may have important implications for exercise training in rehabilitation programs. Although multicenter prospective studies using exercise with high relative intensity to treat patients with the metabolic syndrome are needed to advance our conclusions, we propose that high-intensity exercise training programs may yield more favorable results than programs with low to moderate intensities.
Methods
Patients
Thirty-two patients with the metabolic syndrome defined according to the World Health Organization6 criteria were included. All subjects provided written informed consent, and the regional committee for medical research ethics approved the protocol. Patients were randomized and stratified (by age and gender) into AIT (n=12), CME (n=10), or a control group (n=10). Registration of diet behavior was performed with a questionnaire that was described previously.7
Maximal Oxygen Uptake
Before measurements of maximal oxygen uptake (V̇o2max), subjects were informed about the test and instructed to exercise to their maximum limit. Familiarization with the treadmill, the warmup, and the V̇o2max ramp procedure have been described in detail.8 A leveling off of oxygen uptake despite increased workload and a respiratory exchange ratio >1.05 were used as criteria for reaching the true V̇o2max, and this was achieved in all individuals in the present study.
Training Protocols
Both exercise groups performed endurance training as walking/running "uphill" on a treadmill 3 times a week for 16 weeks. AIT subjects warmed up for 10 minutes at 70% of maximal heart frequency (Hfmax) before performing four 4-minute intervals at 90% of Hfmax with a 3-minute active recovery at 70% of Hfmax between intervals and a 5-minute cool-down period, giving a total exercise time of 40 minutes. To equalize training volumes to similar amounts of kilocalories per session in the 2 groups, the CME group had to exercise for 47 minutes at 70% of Hfmax at each exercise session as previously detailed.8 The control group followed advice from family physicians. Heart frequency was continuously monitored during exercise to ensure that the subjects trained at the intended intensity.
Endothelial Function and Blood Pressure
Endothelial function was measured as flow-mediated dilatation (FMD) with high-resolution vascular ultrasound (14-MHz echo Doppler probe, Vivid 7 System, GE Vingmed Ultrasound, Horten, Norway) according to the current guidelines.9 The procedures for measuring FMD and blood pressure were recently published by our group.10 Shear rate was calculated as blood flow velocity divided by diameter (cm ⋅ s-1 ⋅ cm-1) as previously published.11 Because no group differences were found, data are not presented. All ultrasound images were analyzed in random order with EchoPACtm (GE Vingmed Ultrasound AS) by a blinded investigator.
Biopsies
One week before the V̇o2max test and at least 4 days after the last training session, muscle biopsies of the vastus lateralis muscle were taken with a 5-mm-diameter biopsy needle (Bergstrom, Sweden) under local anesthesia (2% lidocaine). A small muscle sample was used immediately for sarcoplasmic reticulum Ca2+ ATPase 1 and 2 Ca2+ transport measurements with a fluorescence microscope as recently described.12 The remaining biopsy was immediately frozen in liquid nitrogen and stored at -80°C. A fat biopsy was taken with the subjects lying in a prone position by aspiration from the gluteal fat pad with a 20-mL sterile plastic syringe and a 15-gauge needle. Fat samples were immediately frozen in liquid nitrogen and stored at -80°C.
Protein Phosphorylation and Expression Levels
Frozen tissue samples were homogenized in ice-cold lysis buffer (1% Triton X-100 in the presence of phosphatase and protease inhibitors), and the lysates were treated with 100 nmol/L insulin (Sigma, St Louis, Mo) or buffer alone for 10 minutes before phosphorylation in the presence of cold ATP. Proteins were immunoprecipitated with an anti–insulin receptor (IR) antibody (Santa Cruz Biotechnology Inc, Santa Cruz, Calif), analyzed on 7% SDS-PAGE, and transferred to nitrocellulose membranes before detection of phosphorylated residues with a monoclonal anti-phosphotyrosine antibody (Cell Signaling Technology, Danvers, Mass). The blots were reprobed with an antibody against the β subunit of IR (Santa Cruz Biotechnology Inc) to account for the amount of IR in immunopellets. Moreover, 5 μg total lysates was analyzed by 4% to 12% SDS-PAGE and immunoblotting with antibodies against fatty acid transporter protein 1 (FATP-1), fatty acid synthase (FAS), and the peroxisome proliferative–activated receptor γ coactivator 1α (PGC-1α) (Santa Cruz Biotechnology Inc) before reprobing with antibodies against actin or tubulin to normalize for the amount of protein loaded. After incubation with horseradish peroxidase–conjugated anti-immunoglobulin G antibodies, proteins were detected by enhanced chemiluminescence (Amersham Pharmacia Biotech, Uppsala, Sweden) and quantified by densitometry.
Blood Analyses
If not otherwise stated, all blood analyses were performed with standard local procedures. Oxidized low-density lipoprotein (LDL) and adiponectin were measured in plasma with specific Mercodia ELISA kits (Mercodia, Uppsala, Sweden); total nitrite concentration was quantified with a commercially available assay for nitric oxide (NO) detection (R&D Systems, Inc, Minneapolis, Minn); and plasma insulin was analyzed by a radioimmunoassay kit (Linco Research, St Charles, Mich). To estimate β-cell function and overall insulin sensitivity, the homeostasis assessment model (HOMA) was used.
Statistical Analyses
The primary outcome variable was V̇o2max. Prior experience suggests an SD of ≈ 2 to 3.13 No formal sample size calculation was done, but with 10 subjects in each group, a standardized within-group difference of 1.0 may be detected with a paired t test with 80% power at a significance level of 5%.14 Clinically, this corresponds to a detectable difference for V̇o2max of 3 mL ⋅ kg-1 ⋅ min-1. Continuous variables are presented as mean±SD for descriptive purposes and as mean±SEM when group differences are of primary interest. Comparison of continuous variables was done according to design (number of groups, paired or unpaired) using the linear model with correction for baseline value.15 The Wilcoxon, Mann–Whitney, or Kruskal-Wallis nonparametric procedures were used if the assumption of normality and homogeneity of variance was in doubt. Within-group changes in individual risk factors constituting the metabolic syndrome were tested with the McNemar test; the total number of factors changing across groups was tested with the Mann–Whitney test. The potential for spurious significance resulting from multiple testing and a large number of variables relative to subjects is recognized, but because this was a pilot study, no correction for multiple comparisons was applied. Reported probability values are 2 sided and referred to within-group comparison unless specified otherwise. Values of P<0.05 are considered as statistically significant. SPSS 14.0 (SPSS Inc, Chicago, Ill) was used for all analyses.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Baseline Characteristics
At baseline, patients had comparable body weights, body mass indexes, waist-to-hip ratios, blood plasma parameters, and blood pressures (Tables 1 through 3⇓⇓). During the experimental period, 4 patients were unable to complete the training program: 1 (AIT group) relocated, 1 (CME group) withdrew for personal reasons, 1 (control group) had a minor hip injury, and 1 (CME) refused to perform follow-up tests for personal reasons (Figure 1). Thus, 28 patients carried out 90±2% of the scheduled training sessions.
Clinical Follow-Up
Despite no diet alterations during the 16-week intervention period (data not shown), AIT and CME patients exhibited a slight reduction of 3% and 4%, respectively, in body weight (both P<0.05, with no difference between AIT and CME; Table 1) and body mass index (both P<0.05; Table 1). Similarly, the waist circumference was reduced by 5 and 6 cm in the AIT and CME groups, respectively (P<0.05; Table 2). AIT reduced more of the other factors of the metabolic syndrome, including insulin sensitivity (HOMA) (group difference, P<0.05; Tables 2 and 3⇑ ) compared with CME. At the end of the training period, 46% (P<0.05) in the AIT and 37% in the CME groups (P=0.23; between-group difference, P<0.05) were no longer diagnosed with the metabolic syndrome; in the control group, the syndrome persisted in all subjects (Table 3).
Blood Pressure
Both AIT and CME decreased systolic and diastolic blood pressures by ≈ 10 mm Hg (both P<0.05) and ≈ 6 mm Hg (AIT, P<0.05; CME, P=0.24; Table 2), respectively.
Aerobic Capacity
AIT and CME increased V̇o2max by 35% and 16% (P<0.01), respectively (group difference, P<0.01; Figure 2A). Only AIT had a significantly increased peak O2 pulse (P<0.05; between-group difference, P<0.001; Table 2).
PGC-1α and Sarcoplasmic Reticulum Ca2+ ATPase
Decreased exercise capacity in individuals with the metabolic syndrome may be linked to skeletal muscle abnormalities related to mitochondrial biogenesis and excitation-contraction coupling.13,16 Therefore, we examined the levels of PGC-1α, a critical coordinator for activation of metabolic genes controlling substrate use and mitochondrial biogenesis, and the reuptake of Ca2+ into the sarcoplasmic reticulum. We found that PGC-1α levels in the vastus lateralis muscle increased by 138% by AIT but were not significantly altered in the other groups (group difference, P<0.01; Figure 2B). Furthermore, AIT but not CME increased the maximal rates of sarcoplasmic reticulum Ca2+ uptake by ≈ 50% (P<0.03; Figure 2C; between-group difference, P<0.05; Figure 2C).
Endothelial Function
AIT and CME improved FMD by 9% (P<0.001) and 5% (P<0.001), respectively (group difference, P<0.001; Figure 2D). Endothelium-independent dilatation by the administration of nitroglycerine sublingually was equal in all groups (data not shown).
We observed increased availability of NO (36±3%; P<0.03) after AIT but not CME (P=0.37; group difference, P<0.05). Furthermore, oxidized LDL, which negatively regulates the bioavailability of NO, was reduced by 17% (102±8 U/mL before versus 85±7 U/mL after; P<0.001) in AIT but not CME (not shown) (group difference, P<0.01).
Insulin Action in Skeletal Muscle and Fat Tissue
Because IR phosphorylation in skeletal muscle is an excellent surrogate measure of peripheral insulin sensitivity in obese and type 2 diabetic humans,17,18 phosphorylation of the IR β subunit in the presence of insulin was examined. Insulin induced a substantial increase (2.5- to 15-fold; P<0.001; Figure 3A) in receptor phosphorylation (and thus activation) in the vastus lateralis muscle after AIT compared with the other groups (P<0.03). In fat tissue, AIT induced a larger increase in receptor phosphorylation compared with the other groups (P<0.01; Figure 3B).
FATP-1 and FAS
Endurance training did not affect the protein content of FATP-1, a fatty acid transporter, in the vastus lateralis muscle (data not shown). In contrast, FATP-1 levels decreased 3- to 4-fold in fat tissue after AIT but not CME (group difference, P<0.05; Figure 3C). AIT consistently caused a more marked decrease in the protein content of FAS, a key lipogenic enzyme, in white adipose tissue compared with the other groups (P<0.01; Figure 3D), suggesting reduced lipogenesis in this tissue by AIT but not CME.
Blood Analysis
AIT caused a significant improvement in fasting blood glucose compared with the other groups (P<0.05; Table 2). Furthermore, insulin sensitivity and β-cell function appear to be mainly improved by AIT, as indicated by HOMA analysis (group difference, P<0.05; Table 2). Moreover, high-density lipoprotein cholesterol was increased by ≈ 25% after AIT (P<0.05; Table 2) but remained unaltered in the other groups (group difference, P=NS). Consistent with improved insulin sensitivity by AIT, circulating adiponectin, an adipocyte-secreted hormone that is associated with improved insulin sensitivity and amelioration of the metabolic syndrome, was increased (P<0.05; Table 2). Similarly, adiponectin levels were increased by CME (P<0.05; Table 2), perhaps because of a comparable decrease in visceral obesity as suggested by reduced waist circumference in patients undergoing either exercise program (Table 2). Neither training program changed the levels of triglycerides, insulin, microalbuminuria (Table 2), total cholesterol, LDL, C-peptide, or hemoglobin (data not shown).
|
|
| |
| |
|
|
|