| |
Primary Prevention of Atherosclerosis
Time to Take a Selfie? Editorial - Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors
| | | | |
Download the PDF here
Download the PDF here
Download the PDF here
[following this Editorial is full text article and a 3rd report: "Predicting Subclinical Atherosclerosis in Low-Risk Individuals Ideal Cardiovascular Health Score and Fuster-BEWAT Score"
Vijay Nambi and Deepak L. Bhatt - Dec 19 2017 - Journal of the American College of Cardiology
"Normal" blood pressure, "normal" cholesterol, and "no" diabetes are terms that patients are happy to hear at the end of their clinical visit because they consider these terms to be markers of good cardiovascular health. However, what is normal (1,2)? Although guidelines may identify particular levels as "abnormal," dichotomizing continuous variables is always somewhat arbitrary. The expert panels that write guidelines or develop risk scores are clearly familiar with this conundrum, yet due to human fascination with goals and targets, they provide cutpoints. On occasions when they have not, there has been intense debate.
In this issue of the Journal, Fernandez-Friera et al. (3) report that approximately one-half of the 1,779 subjects with no conventional risk factors and on no medications enrolled in the PESA (Progression of Early Subclinical Atherosclerosis) study had subclinical atherosclerosis as detected by ultrasonography or coronary calcium imaging. In this cohort, ∼50% were women, and the mean age was ∼45 years. This analysis of PESA, by including a middle-aged population with no traditional risk factors and not on medications, allowed the investigators to get an unadulterated evaluation of the natural history of atherosclerosis. The authors found that the prevalence of subclinical atherosclerosis increased as low-density lipoprotein cholesterol (LDL-C) increased, but notably, even at an LDL-C concentration between 60 and 70 mg/dl, 11% of the subjects had subclinical atherosclerosis! Interestingly, the iliofemoral arteries had the highest prevalence of plaque, whereas the coronary calcium score identified the least plaque. The investigators also studied a subgroup (n = 740) with optimal risk factors by current standards (blood pressure: <120/80 mm Hg; fasting glucose: <100 mg/dl; hemoglobin A1c: <5.7%; total cholesterol: <200 mg/dl). Plaque was detected in ∼38% of these individuals.
This analysis from the PESA study is an important contribution in our efforts to understand primordial prevention (i.e., stopping the development of risk factors), as well as primary prevention of atherosclerotic cardiovascular disease (ASCVD). The study showed that, even in a group that had reasonable primordial prevention, the prevalence of subclinical atherosclerosis was significant. The PESA study used a comprehensive, multi-bed, and clinically implementable noninvasive assessment of subclinical atherosclerosis that included computed tomography-based coronary calcium scoring and ultrasonography-based assessment of plaque in the infrarenal aorta and iliofemoral and carotid arteries. Thus, these data extend previous autopsy studies and invasive intravascular ultrasonography evaluations that had shown a high prevalence of atheroma at young ages (4) but accomplished this evaluation noninvasively, signifying a major conceptual advance.
Our definitions of "normal" or "optimal" for various risk factors have continued to evolve with science and are geared to predict the risk of ASCVD events and to provide goals for the management of ASCVD, not to predict the development of "subclinical" atherosclerosis. For example, the efficacy of statins in both primary and secondary prevention of ASCVD led to revising "optimal" values of cholesterol in both healthy and diseased patients (5). More recently, further intensive LDL-C-lowering (from ∼70 mg/dl to ∼50 mg/dl) when ezetimibe was added to statin therapy was shown to reduce incident ASCVD (6). Similarly, the addition of PCSK-9 inhibitors favorably altered atheroma on intravascular ultrasound imaging (7) and reduced ASCVD events while achieving an LDL-C of ∼30 mg/dl (8). All these data have resulted in a discussion of how low the LDL-C target should be in the secondary prevention of ASCVD. In this context, the current analysis by Fernandez-Friera et al. (3) is highly relevant and now raises the question of what the optimal cholesterol level may be in the primordial and primary prevention of ASCVD (1,2,9,10).
Furthermore, cholesterol is not the only conventional risk factor for which the optimal level is not clear. For example, the targets and goals for management of both blood pressure and blood glucose concentration have been the subject of much discussion. Identification of higher risk subgroups in whom the benefit-to-risk ratio may be favorable could be a strategy to consider. Hence, the incremental value of biomarkers such as troponin in gauging risk has been evaluated and demonstrated in primary and secondary prevention populations (11-14). Very recently, the inflammatory axis has also been shown to influence atherosclerotic risk in a modifiable manner (15-17). "Lower" may indeed be better for cholesterol, blood pressure, glucose, and now inflammation as well. However, how low to go and how to get there for all these different axes of risk without provoking side effects may be the key challenge in personalizing therapies.
Perhaps, arterial imaging at an early stage could help guide such an algorithm (Figure 1). Potentially, combining information from noninvasive imaging with biomarkers and genetic markers would help further refine and personalize risk assessment prior to the establishment of atherosclerosis and/or ASCVD. Targeted intensive lifestyle efforts for primordial prevention and targeted pharmacotherapy (such as a polypill strategy) for selected primary prevention of ASCVD may be the answer. However, long-term randomized trials will be necessary, as will cost-effectiveness analyses. In the meantime, physicians should consider avoiding the term "normal," and instead, inform their patients of their current cholesterol, blood pressure, or blood glucose values and discuss what preventive efforts may be needed given their level of risk.
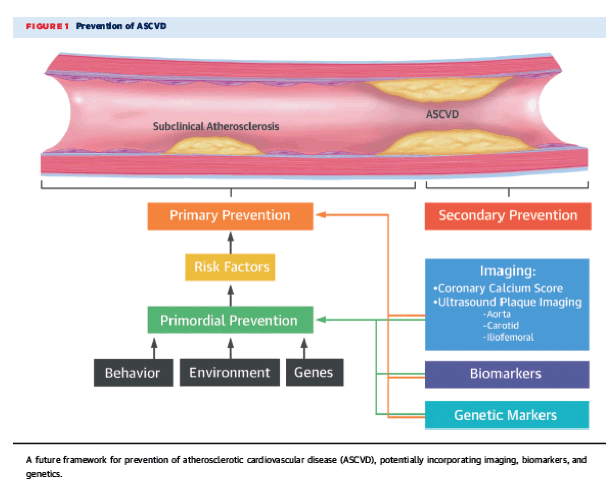
Figure 1
Prevention of ASCVD
A future framework for prevention of atherosclerotic cardiovascular disease (ASCVD), potentially incorporating imaging, biomarkers, and genetics.
In summary, this elegant analysis from PESA (3) demonstrates that subclinical atherosclerosis is highly prevalent, even in individuals with "normal" values for conventional cardiovascular risk factors, bringing into question the definition of normal in primordial and primary prevention and, for that matter, the definition of primordial prevention itself. The management of ASCVD has conventionally focused on secondary prevention or short-term (∼10-year) primary prevention. The findings from the current analysis underscore the need to start using advances in imaging, biomarkers, and genetics to re-examine the definition of "optimal" cardiovascular health. Given the significant lead time that atherosclerosis affords us, as a community, it may be time to take a "selfie" of not only our arteries but also our approaches to primordial and primary prevention of ASCVD.
------------------
Predicting Subclinical Atherosclerosis in Low-Risk Individuals
Ideal Cardiovascular Health Score and Fuster-BEWAT Score
Juan Miguel Fernandez-Alvira, Valentin Fuster, Stuart Pocock, Javier Sanz, Leticia Fernandez-Friera, Martin Laclaustra, Rodrigo Fernandez-Jimenez, Jose Mendiguren, Antonio Fernandez-Ortiz, Borja Ibañez and Hector Bueno
Abstract
Background The ideal cardiovascular health score (ICHS) is recommended for use in primary prevention. Simpler tools not requiring laboratory tests, such as the Fuster-BEWAT (blood pressure [B], exercise [E], weight [W], alimentation [A], and tobacco [T]) score (FBS), are also available.
Objectives The purpose of this study was to compare the effectiveness of ICHS and FBS in predicting the presence and extent of subclinical atherosclerosis.
Methods A total of 3,983 participants 40 to 54 years of age were enrolled in the PESA (Progression of Early Subclinical Atherosclerosis) cohort. Subclinical atherosclerosis was measured in right and left carotids, abdominal aorta, right and left iliofemoral arteries, and coronary arteries. Subjects were classified as having poor, intermediate, or ideal cardiovascular health based on the number of favorable ICHS or FBS.
Results With poor ICHS and FBS as references, individuals with ideal ICHS and FBS showed lower adjusted odds of having atherosclerotic plaques (ICHS odds ratio [OR]: 0.41; 95% confidence interval [CI]: 0.31 to 0.55 vs. FBS OR: 0.49; 95% CI: 0.36 to 0.66), coronary artery calcium (CACS) ≥1 (CACS OR: 0.41; 95% CI: 0.28 to 0.60 vs. CACS OR: 0.53; 95% CI: 0.38 to 0.74), higher number of affected territories (OR: 0.32; 95% CI: 0.26 to 0.41 vs. OR: 0.39; 95% CI: 0.31 to 0.50), and higher CACS level (OR: 0.40; 95% CI: 0.28 to 0.58 vs. OR: 0.52; 95% CI: 0.38 to 0.72). Similar levels of significantly discriminating accuracy were found for ICHS and FBS with respect to the presence of plaques (C-statistic: 0.694; 95% CI: 0.678 to 0.711 vs. 0.692; 95% CI: 0.676 to 0.709, respectively) and for CACS ≥1 (C-statistic: 0.782; 95% CI: 0.765 to 0.800 vs. 0.780; 95% CI: 0.762 to 0.798, respectively).
Conclusions Both scores predict the presence and extent of subclinical atherosclerosis with similar accuracy, highlighting the value of the FBS as a simpler and more affordable score for evaluating the risk of subclinical disease.
--------------------
Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors
Leticia Fernandez-Friera, Valentin Fuster, Beatriz Lopez-Melgar, Belen Oliva, Jose M. Garcia-Ruiz, Jose Mendiguren, Hector Bueno, Stuart Pocock, Borja Ibañez, Antonio Fernandez-Ortiz and Javier Sanz
Abstract
Background Absence of cardiovascular risk factors (CVRFs) is traditionally considered low risk for atherosclerosis; however, individuals without CVRFs, as currently defined, still have events.
Objectives This study sought to identify predictors of subclinical atherosclerosis in CVRF-free individuals.
Methods Participants from the PESA (Progression of Early Subclinical Atherosclerosis) study (n = 4,184) without conventional CVRFs were evaluated (n = 1,779; 45.0 ± 4.1 years, 50.3% women). CVRF freedom was defined as no current smoking and untreated blood pressure <140/90 mm Hg, fasting glucose <126 mg/dl, total cholesterol <240 mg/dl, low-density lipoprotein cholesterol (LDL-C) <160 mg/dl, and high-density lipoprotein cholesterol ≥40 mg/dl. A subgroup with optimal CVRFs (n = 740) was also defined as having blood pressure <120/80 mm Hg, fasting glucose <100 mg/dl, glycosylated hemoglobin <5.7%, and total cholesterol <200 mg/dl. We evaluated ultrasound-detected carotid, iliofemoral, and abdominal aortic plaques; coronary artery calcification; serum biomarkers; and lifestyle. Adjusted odds ratios (with 95% confidence interval) and ordinal logistic regression models were used.
Results Subclinical atherosclerosis (plaque or coronary artery calcification) was present in 49.7% of CVRF-free participants. Together with male sex and age, LDL-C was independently associated with atherosclerosis presence and extent, in both the CVRF-free and CVRF-optimal groups (odds ratio [x10 mg/dl]: 1.14 to 1.18; p < 0.01 for all). Atherosclerosis presence and extent was also associated in the CVRF-free group with glycosylated hemoglobin levels.
Conclusions Many CVRF-free middle-aged individuals have atherosclerosis. LDL-C, even at levels currently considered normal, is independently associated with the presence and extent of early systemic atherosclerosis in the absence of major CVRFs. These findings support more effective LDL-C lowering for primordial prevention, even in individuals conventionally considered at optimal risk. (Progression of Early Subclinical Atherosclerosis [PESA] Study; NCT01410318)
Cardiovascular risk in asymptomatic individuals is assessed on the basis of conventional cardiovascular risk factors (CVRFs), and most cardiovascular events are linked to elevated CVRFs (1). However, atherosclerosis and cardiovascular events are common even among individuals with a low CVRF burden (2-4). This is especially the case among younger adults and women, who can experience cardiovascular events despite being considered at low short-term risk (5-7). According to current preventive recommendations (8), healthy individuals without CVRFs (as presently defined) are usually not considered a target for prevention strategies despite the possible presence of atherosclerosis.
Subclinical atherosclerosis underlies most cardiovascular events, and its detection can improve risk stratification (9,10). However, a mismatch has been reported between low conventional risk and the presence of subclinical atherosclerosis detected by coronary artery calcification (CAC) or carotid ultrasound (11,12). Our group identified subclinical atherosclerosis in nearly 60% of middle-aged individuals classified at low risk according to traditional risk scales, with multiple vascular sites affected in 41% (13). These findings demonstrate a disparity between conventional CVRFs and the presence of atherosclerosis, suggesting that other factors play a role in atherogenesis. In this study, we aimed to explore and identify potential predictors of the presence and multiterritorial extent of subclinical atherosclerosis in the absence of major CVRFs.
Discussion
Absence of conventional CVRFs is traditionally considered a reliable indicator of low atherosclerosis risk. However, we identified subclinical atherosclerosis in one-half of a CVRF-free middle-aged population, with multiple vascular sites affected in nearly 30%. This finding expands on previous studies examining the association of risk with subclinical disease in single vascular territories (4,12), characterizing the extent of systemic atherosclerosis through multiterritorial assessment in low-risk individuals. Moreover, more than one-third of individuals with optimal CVRF levels and an ostensibly healthy status had subclinical atherosclerosis, suggesting that additional, poorly defined factors play a role in early atherogenesis. In the absence of conventional CVRFs, the presence and extent of atherosclerosis were associated with age, male sex, LDL-C, and HbA1c. Even with CVRF levels considered to be optimal, atherosclerotic burden remained independently associated with age, male sex, and LDL-C, highlighting the crucial role of LDL-C in early disease stages.
The LDL-C hypothesis considers LDL-C as a causal factor in atherosclerosis. Although this hypothesis is generally accepted, controversy remains regarding its validity (28,29).
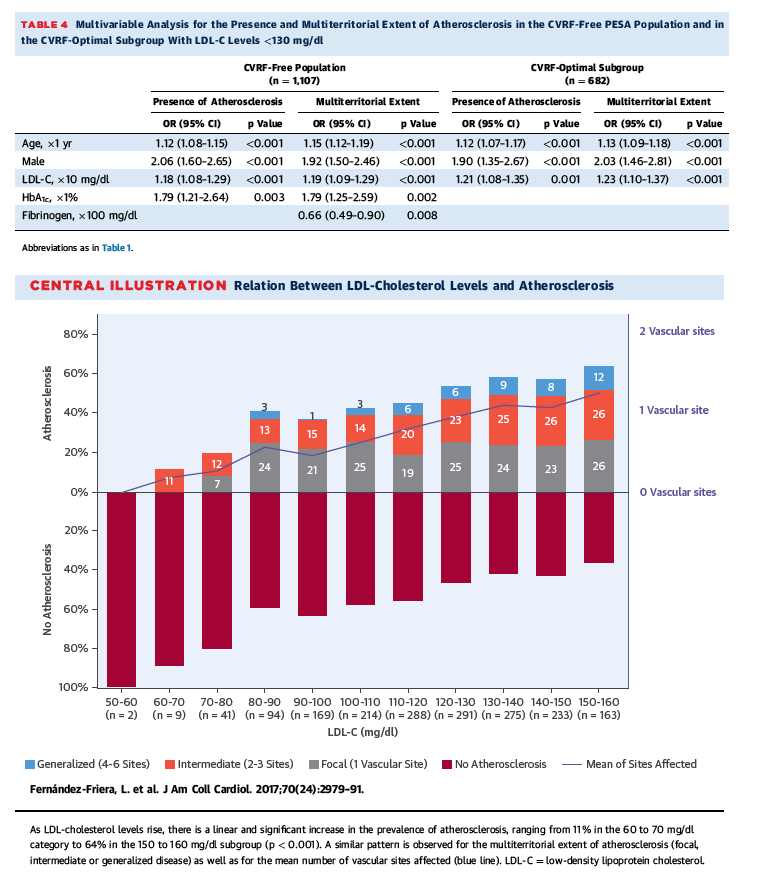
Evidence supporting this hypothesis stems from experimental models, epidemiological cohorts, and cholesterol-lowering (mainly statin-based) clinical trials (30). However, potential remaining confounders should be considered. Animals used in experimental models typically develop much higher concentrations of plasma cholesterol than seen clinically (31), and results need to be extrapolated to humans. Clinical studies have typically enrolled participants with either clearly abnormal lipid levels or coexisting CVRFs, which may have synergistic or additive effects on disease development (32). In addition, some benefits of statin therapy may be related to pleiotropic effects beyond cholesterol lowering (30). In this study of apparently healthy individuals without conventional CVRFs, we demonstrated an independent and direct link between LDL-C levels and atherosclerotic burden. In fact, LDL-C was the strongest modifiable factor associated with atherosclerosis. Furthermore, even when all other risk factors were at optimal levels, this association persisted. Although association does not equate with causation, in the context of extensive prior data, we believe that these unique data from a large human cohort eliminate some of the potential confounders mentioned previously and provide indirect but solid evidence for the central role of LDL-C in early human atherogenesis. This is further highlighted by our findings of associations between LDL-C and disease in participants with low 30-year risk. These results also support the notion that cholesterol alone, in the absence of other known conventional CVRFs, may be enough to drive the development of atherosclerosis in humans (33). Multivariable analysis yielded similar results when LDL-C was replaced with non-HDL-C, suggesting no advantage of using one lipid variable over the other. Conversely, the multivariable models showed no link to subclinical atherosclerosis for other apolipoprotein B-containing particles, specifically oxidized LDL-C and lipoprotein (a). Although these particles are in principle more atherogenic, the absence of association may be related to their low concentrations and the low between-group variability in this CVRF-free population, and their role may be more important in the setting of higher cardiovascular risk (34). Importantly, LDL-C levels in our population were well within the range considered normal, reinforcing the concept that desirable LDL concentrations are probably much lower than those currently recommended (35). If confirmed, the results shown in the Central Illustration and Figure 4 would indicate that atherosclerosis in both men and women develops above an LDL-C threshold concentration of approximately 50 to 60 mg/dl, similar to the level associated with disease regression (36). This hypothesis is consistent with recent lipid-lowering trials, in which adverse clinical outcomes were significantly reduced when LDL-C levels were lowered below current targets (37). Thus, our findings may have important implications for primordial prevention strategies and for establishing cutoff values to define lipid disorders.
Additional independent (and nonmodifiable) predictors of atherosclerosis included age and sex. It is known that age is the most significant risk factor for cardiovascular events (8), and we also showed a strong association with subclinical atherosclerosis. This is likely related to the longer exposure to a variety of atherosclerotic risk determinants (e.g., LDL-C) as well as other aging-associated phenomena such as increased nucleic acid damage, apoptosis, or reduced regenerative capacity (38). Sex-based differences in atherosclerosis prevalence and severity are also well described, with women experiencing their first clinical event a decade later than men on average (39). Underlying mechanisms are incompletely understood but are probably multifactorial, including estrogen levels as well as differences in risk factor prevalence and susceptibility, psychosocial factors, and vascular biology (40).
In our study, HbA1c was also independently associated with the presence and extent of subclinical atherosclerosis in the absence of CVRFs, both for participants with LDL-C <160 and <130 mg/dl, but not in those participants with optimal CVRF levels. HbA1c reflects average glucose levels in the previous 2 to 3 months and therefore provides an index of glucose metabolism. Several previous reports have demonstrated an association between HbA1c and subclinical atherosclerosis. In 2,340 nondiabetic individuals, higher HbA1c concentrations (between 5.7% and 6.4%) were independently associated with increased CAC and carotid intima-media thickness (41). In another nondiabetic prospective series (n = 2,652), the upper 2 quartiles of HbA1c levels (>5.7%) were linked to both carotid intima-media thickness progression and cardiovascular events (42). Notably, in our nondiabetic population we also observed a relative increase in systemic disease burden at HbA1c levels >5.7% (Figure 3). These findings suggest that slightly increased HbA1c levels are linked to subclinical atherosclerosis, particularly in combination with other risk factors (43), possibly explaining the increased cardiovascular risk associated with pre-diabetes (44). Although again our data do not establish a causal role, extensive prior evidence indicates detrimental vascular effects of chronic hyperglycemia through a variety of mechanisms (43).
Other serum markers possibly associated with the multiterritorial extent of atherosclerosis included VCAM-1 (an endothelial adhesion protein), cystatin C (an endogenous marker of renal function) (45), and fibrinogen (a hemostatic and inflammatory marker). Although each of these markers has potential mechanistic links to atherosclerosis, we did not find consistent associations in our models, and their link to atherosclerosis in the absence of CVRFs needs further investigation. Interestingly, blood pressure was not associated with systemic atherosclerosis in our sample, although we found a link with carotid disease (Online Table 9). We observed a paradoxical response between subclinical disease and healthy lifestyle parameters, such as diet and physical activity. Whether this unexpected finding reflects a real biological process or the methodology used to measure these complex and multifactorial parameters is beyond the scope of this paper, and the influence of lifestyle behaviors has been addressed in recent PESA publications (24). We hypothesize that early atherogenesis in the absence of CVRFs and even with optimal risk factors levels is driven by age, sex, and LDL-C levels currently considered normal. Mild elevations in HbA1c and advanced glycation products (associated with glucose metabolic dysregulation and aging) may cause further vascular injury. Although our data support the central role of LDL-C in early atherosclerosis, they do not exclude potential contributions from other multiple factors, which might be demonstrated with large sample sizes. Thus, early control of all risk factors should be taken into consideration for primordial prevention.
Our findings need to be placed in the context of the CVRF definitions used here. Specifically, the definition of dyslipidemia is not universally accepted and different lipid thresholds may slightly alter the results. However, we employed a commonly used definition from the National Cholesterol Education Program guidelines (16), in line with previous PESA study publications (13). To test the consistency of our results, we performed a secondary analysis including LDL-C levels <130 mg/dl, obtaining comparable results. Similarly, there is no consensus definition of an optimal risk profile. Our definition of optimal risk individuals is largely based on widely accepted factors of ideal cardiovascular health (3); however, our selection criteria did not include ideal cardiovascular behaviors other than smoking because only 121 of 1,779 individuals in our cohort reached the ideal levels for all 7 metrics. This very low ideal-health prevalence, which is consistent with previous reports (46), precluded meaningful statistical analysis. Recent U.S. guidelines (19) propose different optimal CVRF thresholds, but only 97 (5.5%) participants in our study fulfilled these criteria (not shown), again precluding meaningful multivariable analysis. Similarly, we could not perform multivariable analysis using a more restrictive LDL-C cutoff (<100 mg/dl) because of insufficient power (n = 235, not shown). In any case, our findings strongly suggest that thresholds to define elevated LDL-C should be lower than recommended across current guidelines.
Study limitations
In the PESA study, diabetes was diagnosed based on glucose levels and not on HbA1c levels; the study population therefore included a small proportion (0.17%) of participants with HbA1c concentrations >6.4 mg/dl who could qualify as having diabetes but were not excluded. However, a sensitivity analysis excluding these participants yielded similar results (not shown). The small number of participants with LDL-C <70 mg/dl precludes reaching strong conclusions about a potential LDL-C threshold below which disease does not develop; however, the linear trend observed across high LDL-C levels supports the possibility of such a threshold. We did not evaluate other nonmodifiable risk factors (e.g., second-hand smoking or air pollution) and did not explore the potential roles of diet and exercise in greater detail because this was not the focus of this study. However, diet and physical activity showed no significant associations with atherosclerosis in the main models used (not shown), probably due to the homogeneity of these variables in our sample. Similarly, we did not evaluate all possible serum biomarkers because they were not included in the baseline PESA study examination (4). Finally, we did not evaluate the genetic contribution to disease development (47,48), which can be independent of CVRFs and could thus play an important role in our population.
Conclusions
Subclinical atherosclerosis is present in one-half of middle-aged PESA study individuals without major CVRFs and in one-third of those in the CVRF-optimal subgroup, suggesting that additional factors are involved in its development. LDL-C, at levels currently considered normal, is independently associated with the presence and extent of atherosclerosis in this setting, including in those participants with optimal risk profile. Thus, these data provide strong evidence of a unique, independent role of LDL-C in early human atherogenesis. These findings have important implications for guiding primordial prevention and understanding the mechanisms underlying early atherosclerosis.
Perspectives
COMPETENCY IN MEDICAL KNOWLEDGE: Subclinical atherosclerosis can be detected in about one-half of otherwise healthy, middle-aged individuals without conventional cardiovascular risk factors. Serum LDL-C levels, even within the range currently considered normal, is independently associated with the presence and extent of subclinical atherosclerosis in multiple vascular territories.
TRANSLATIONAL OUTLOOK: The high global cardiovascular burden of cardiovascular disease makes effective primordial prevention a health care priority. Prospective studies are needed to evaluate the efficacy of more aggressive LDL-C lowering strategies at both the individual and population levels to reduce the incidence of clinical ischemic events.
Methods
Study design
This study was conducted in a subset of individuals from the PESA (Progression of Early Subclinical Atherosclerosis) study (13-15) with CVRF levels below current thresholds. The PESA study uses noninvasive imaging to prospectively evaluate the presence and progression of subclinical atherosclerosis in a middle-aged population of 4,184 adults aged between 40 and 54 years. The main exclusion criteria were known cardiovascular disease, active treatment for cancer, or any disease expected to decrease life expectancy or protocol adherence. Participants underwent clinical interviews, physical activity and lifestyle evaluations, physical examination, electrocardiogram, laboratory analysis, and imaging studies at baseline, with repeat evaluations scheduled for 3- and 6-year follow-up visits. The study protocol was approved by the Instituto de Salud Carlos III Ethics Committee and all participants provided written informed consent.
Definition of the CVRF-free population and the CVRF-optimal subgroup
This study included nonsmokers with no hypertension, diabetes, or dyslipidemia according to Adult Treatment Panel III CVRF definitions (16,17): 1) untreated systolic blood pressure <140 mm Hg and diastolic blood pressure <90 mm Hg; 2) untreated fasting plasma glucose <126 mg/dl; 3) untreated total cholesterol <240 mg/dl, low-density lipoprotein cholesterol (LDL-C) <160 mg/dl, and high-density lipoprotein cholesterol (HDL-C) ≥40 mg/dl; and 4) no current smoking status. This subpopulation represents 42.5% of the total PESA study population (Figure 1).
Within the conventional CVRF-free population, we also defined a subgroup of individuals with optimal modifiable CVRFs (3,18): systolic blood pressure <120 mm Hg, diastolic blood pressure <80 mm Hg, total cholesterol <200 mg/dl, fasting plasma glucose <100 mg/dl, and glycosylated hemoglobin (HbA1c) <5.7%.
Assessment of CVRFs, serum biomarkers, and lifestyle parameters
CVRFs were prospectively collected through questionnaires (smoking, family history) or objective quantification (hypertension, diabetes, dyslipidemia) as previously described (13). Family history of cardiovascular disease was defined as having a first-degree relative diagnosed with clinical atherosclerosis below 55 years of age in men and 65 years of age in women (16). Obesity was defined as body mass index ≥30 kg/m2 (2,16). The 10-year risk of atherosclerotic cardiovascular disease was calculated using the Pooled Cohort Equations and cutoffs were defined as <5%, 5% to <7.5%, and ≥7.5% for low, intermediate, and high risk, respectively (19). The 30-year Framingham risk score was also measured and classified as low (<10%), moderate (10% to 20%), or high (<20%) risk (20).
Venous blood was collected after 8 h of fasting and samples were tested for total cholesterol, HDL-C, LDL-C, oxidized LDL-C, triglycerides, lipoprotein (a), glucose, insulin, HbA1c, cystatin C, and creatinine by standard methods (14). LDL-C was calculated by the Friedewald method except for participants with triglycerides >300 mg/dl, where it was measured directly. The estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration equation (21). The baseline PESA study protocol also included the following inflammation markers: high-sensitivity C-reactive protein (hs-CRP), fibrinogen, vascular cell adhesion molecule (VCAM)-1, and P-selectin. Physical activity was assessed by triaxial accelerometry with ActiTrainer accelerometers (ActiGraph, Pensacola, Florida) placed on each participant's waist for 7 consecutive days, including sleep time. Moderate and vigorous physical activity were defined according to standard Troiano cutoffs (22). We also calculated the PREDIMED (PREvencion con DIeta MEDiterranea) score, which reflects increasing adherence to Mediterranean diet (23,24). In addition, 7 ideal cardiovascular health metrics were quantified, as recently proposed (3).
Assessment of subclinical atherosclerosis
Two-dimensional vascular ultrasound and noncontrast cardiac computed tomography were performed in all participants as previously described (13). In brief, presence of atherosclerotic plaques by ultrasound was assessed by cross-sectional sweep of carotids, infrarenal abdominal aorta, and iliofemoral arteries. Plaques were defined as focal protrusions into the arterial lumen of thickness >0.5 mm or >50% of the surrounding intima-media thickness, or as a diffuse intima-media thickness >1.5 mm (25). The CAC score was calculated from computed tomography images by the Agatston method (26). All images were analyzed at a central Imaging Core Laboratory by experienced, blinded operators.
Subclinical atherosclerosis was defined as the presence of atherosclerotic plaques by vascular ultrasound or CAC score ≥1. The multiterritorial extent of subclinical atherosclerosis was defined according to the number of vascular sites with evidence of disease, including right carotid, left carotid, abdominal aorta, right iliofemoral, left iliofemoral, and coronary arteries. Participants were classified as disease free (0 vascular sites affected) or having focal (1 site), intermediate (2 to 3 sites), or generalized atherosclerosis (4 to 6 sites) (13).
Statistics
The distribution of continuous variables was analyzed using graphical methods. Log transformation was performed before analyses to normalize the distribution as appropriate. Comparisons between participants with and without atherosclerosis were performed using a chi-square test for categorical variables and the Student's t-test for continuous variables. Linear trends across groups according to multiterritorial extent were evaluated with an extension of the nonparametric Wilcoxon rank sum test (27). Logistic and ordinal regression models with forward stepwise variable selection were used to analyze the associations of multiple covariates with the presence and extent of atherosclerosis in the CVRF-free and CVRF-optimal groups. Analyses were then repeated with inclusion restricted to participants with LDL-C <130 mg/dl. Candidate variables with a clinical rationale explored in the multivariate analyses included age, sex, body mass index, systolic blood pressure, diastolic blood pressure, family history of premature cardiovascular disease, fasting glucose, insulin, HbA1c, triglycerides, HDL-C, LDL-C, oxidized LDL-C, lipoprotein (a), eGFR, cystatin C, hs-CRP, VCAM-1, P-selectin, and fibrinogen. Weight, height, obesity, total cholesterol, and risk scores were excluded due to multicollinearity, defined as a correlation r ≥0.8 between variables. To better describe the association between the identified independent risk factors and the multiterritorial extent of atherosclerosis, ordinal logistic regression models were replicated after categorizing the index variable into quintiles or 3 groups for age (40 to 44, 45 to 49, and 50 to 54 years of age). Associations were expressed as odds ratio (OR) and standardized OR with 95% confidence interval (CI). Statistical analyses were conducted using Stata version 12 (StataCorp, College Station, Texas). A p value < 0.05 was considered statically significant.
Results
Characterization of the CVRF-free PESA study population: Mismatch with atherosclerosis
Our study population consisted of 1,779 individuals (50.3% women, 45.0 ± 4.1 years of age), with most in the 40 to 44 years of age subgroup (51.5% vs. 31.4% and 17.1% in the 45 to 49 and 50 to 54 years of age subgroups, respectively). As expected, the majority of individuals (94.6%) had low 10-year cardiovascular risk; intermediate and high risk were observed in 56 (3.1%) and 10 (0.6%) participants, respectively. The corresponding long-term risk proportions were 54.6%, 35.6% and 9.8%. Online Table 1 shows baseline characteristics of the study population (CVRF free) and of the PESA participants with CVRFs.
Tables 1 and 2 summarize baseline characteristics, serum biomarkers, and lifestyle parameters of study participants stratified according to the presence and extent of atherosclerosis. All CVRFs, risk scores, and lifestyle measurements except for family history differed significantly according to atherosclerosis status. Similarly, significant differences were found in all serum biomarkers except for lipoprotein (a), cystatin C, hs-CRP, and fibrinogen. Ideal cardiovascular health metrics are shown in Online Table 2. As expected in this CVRF-free population, ideal metrics were prevalent, although only 121 (6.8%) participants met all 7 ideal criteria. Significant differences in these health metrics between those with and without atherosclerosis were found for blood pressure, total cholesterol, glucose, and body mass index, but not for smoking, physical activity, and diet.
Despite the absence of conventional CVRFs, subclinical atherosclerosis was highly prevalent (49.7%). Overall, 46.7% had peripheral atherosclerotic plaques: 22.7% in the carotid arteries, 17.2% in the infrarenal aorta, and 30.1% in the iliofemoral arteries. CAC was detected in 11.1% of participants, the majority of them with mild calcification (183 individuals with a CAC score <100, 14 with a score of 100 to 399, and 1 with a score ≥400). Analysis of the extent of atherosclerosis revealed focal disease in 22.6% of participants, intermediate disease in 20.9%, and generalized disease in 6.0%. Among participants with optimal CVRFs (n = 740) (Online Tables 3 and 4), 280 (37.8%) had atherosclerosis, with peripheral plaques in 268 individuals and CAC in 43. In this subgroup, focal, intermediate, and generalized atherosclerosis was present in 20.8%, 13.8%, and 3.2% of participants, respectively.
Predictors of atherosclerosis presence and multiterritorial extent
In the CVRF-free population, univariable analyses showed significant associations between disease presence and extent and all measured variables except for family history of cardiovascular disease, lipoprotein (a), cystatin C, hs-CRP, and fibrinogen. VCAM-1 was associated with the extent but not the presence of atherosclerosis (Online Tables 5 and 6). In multivariable models, male sex, age, LDL-C, and HbA1c were associated with the presence of disease (Table 3). Age and sex showed the strongest associations with atherosclerosis presence, followed by LDL-C (Figure 2). The same variables, and additionally VCAM-1 and cystatin C, were also associated with multiterritorial extent of atherosclerosis (Table 3). Figure 3 shows the stratification of these associations according to age intervals (40 to 44, 45 to 49, and 50 to 54 years) by sex, and to quintiles for LDL-C, HbA1c, VCAM-1, and cystatin C. Again, age and sex demonstrated the strongest associations with atherosclerosis multiterritorial extent, followed by LDL-C (Figure 3). When restricting analyses to participants with atherosclerosis, age, LDL-C, VCAM-1, and systolic blood pressure were associated with increasing disease extent in the CVRF-free group, and only age and triglycerides in the CVRF-optimal group; however, sample size in the latter analysis was too small (Online Table 7).
In the subgroup with optimal CVRFs, age, male sex, and LDL-C were the only variables significantly associated with both disease presence and multiterritorial extent (Table 3). Similar to the overall population, age and male sex had the strongest associations with the presence and extent of atherosclerosis (Figure 2).
In both the CVRF-free and CVRF-optimal groups, results were similar when LDL-C was replaced by non-HDL-C (Online Table 8).
Predictors of atherosclerosis in participants with LDL-C <130 mg/dl
Similar results were obtained in a further multivariable analysis restricted to individuals with LDL-C <130 mg/dl (1,107 CVRF free and 682 CVRF optimal). Age, male sex, and LDL-C were the only variables associated with atherosclerosis presence and multiterritorial extent in both groups. In the CVRF-free group only, HbA1c was also associated with both atherosclerosis presence and extent, and fibrinogen with extent (Table 4).
"Normal" LDL-C values are independently associated with subclinical atherosclerosis
The relationship between LDL-C and atherosclerosis in the absence of dyslipidemia, hypertension, diabetes, and smoking is illustrated in the Central Illustration and Figure 4. As LDL-C levels increased, there was a linear and significant increase in the prevalence of atherosclerosis, ranging from 11% in the 60 to 70 mg/dl category to 64% in the 150 to 160 mg/dl subgroup (p < 0.001) (Central Illustration). This progressive increase was noted in both men and women (Online Figure 1). A similar pattern was observed for the number of vascular sites affected (Central Illustration) and for each vascular bed analyzed separately (Figure 4). Indeed, in a secondary analysis by each vascular territory, LDL-C remained associated with atherosclerosis presence in each territory for the total CVRF-free population (Online Table 9).
Finally, we also assessed whether LDL-C tracked atherosclerosis similarly across 30-year Framingham risk score categories. Whereas there was influence in the CVRF-free population for presence and multiterritorial atherosclerosis (interaction test p = 0.035 and p = 0.005, respectively), this was absent in the CVRF-optimal subgroup (p = 0.217 and p = 0.344, respectively). To observe the effects of this interaction, we performed a stratified multivariable analysis by each 30-year Framingham risk score category. LDL-C remained significantly associated with subclinical atherosclerosis only in the low-risk group (Online Table 10).
_______________________________
|
| | | | | | |
|