 |
 |
 |
| |
Weekly Oral MK-8591 Protects Male Rhesus Macaques against Repeated Low Dose Intrarectal Challenge with SHIV109CP3
|
| |
| |
Reported by Jules Levin
9th IAS Conference on HIV Science (IAS 2017), July 23-26, 2017, Paris
Martin Markowitz MD
Aaron Diamond AIDS Research Center
An affiliate of the Rockefeller University
New York, New York
USA
Antiviral Activity of EFdA [MK-8591] Against NRTI-Sensitive and -Resistant Strains of HIV-2 - (02/24/17)
MK-8591 Concentrations at Sites of HIV Transmission and Replication - (02/23/17)
EFdA / MK-8591 - HIV pre-exposure prophylaxis for women and infants prevents vaginal and oral HIV transmission in a preclinical model of HIV infection - (08/05/16)
Pre-exposure Prophylaxis with EFdA Offers Strong Protection against High Dose Mucosal HIV Challenges - [Long-Acting New NRTI for Treatment & PrEP] - (10/24/16)
Efficacy of once-weekly MK-8591 in SIV infected rhesus macaques - (06/14/15)
CROI: Long-Acting Oral and Parenteral Dosing of MK-8591 for HIV Treatment or Prophylaxis - (02/24/16)
CROI: A Single Monotherapy Dose of MK-8591,a Novel NRTI, Suppresses HIV for Ten Days - (02/24/16)

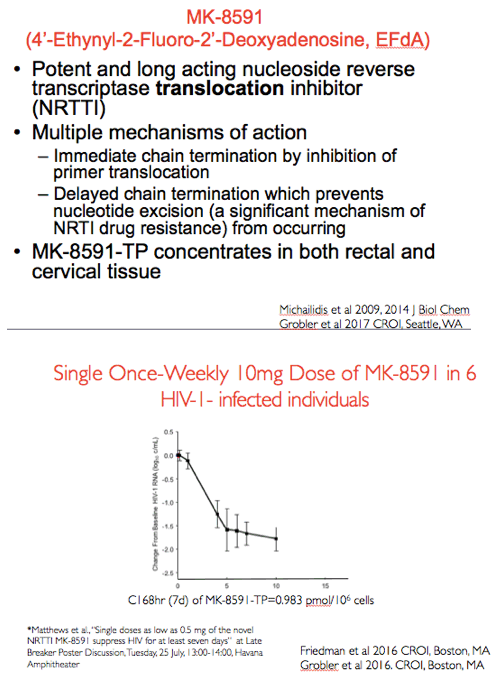
As shown in the figure on the left with days on the x axis and change in HIV RNA from baseline on the y axis a single dose of 10mg of MK-8591 orally in 6 HIV infected individuals resulted in a robust decrease in plasma viremia. This was associated with a trough level of approximately 1pmol/10^6 cells at day 7
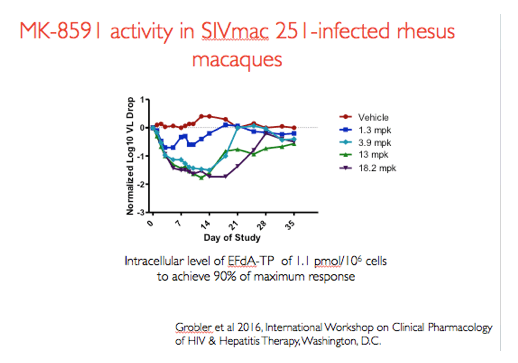
In a previous dose ranging experiments in rhesus macaques infected with SIVmac251 animals were administered 2 weekly oral doses of MK-8591 in increasing doses as shown in this figure. Doses of 3.9mg/kg and above were associated with maximal viral load decline and an intracellular level of EFdA-TP of 1.1 pmol/10^6 cells was found to achieve a maximal EC90. These data form the basis for the dose of MK-8591 used in the challenge experiment I will describe.
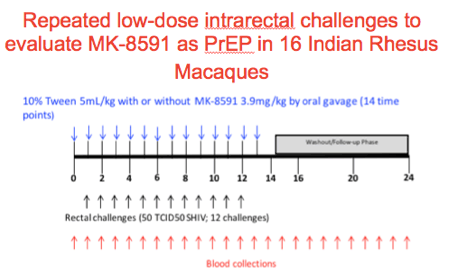
We performed the following experiment to evaluate MK-8591 as PrEP in 16 Indian rhesus macaques.
Animals were treated on day 1 and weekly thereafter with 5ml/kg of 10%Tween80 with or without MK-8591 3.9mg/kg by oral gavage up to 14 times.
At baseline, day 6 and weekly thereafter until week 24 blood was drawn for viral, immune and PK evaluations
On day 6 animals were challenged with 50 TCID50 of SHIV intrarectally until either infection was documented or up to a total of 12 challenges.
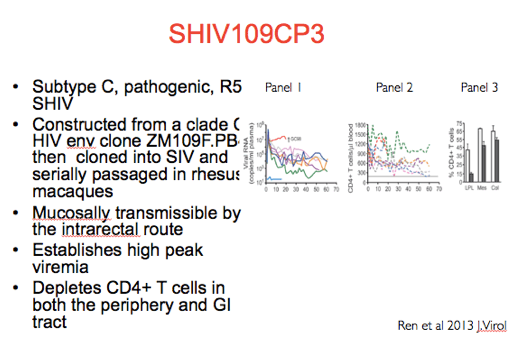
In this particular challenge experiment we used SHIV109CP3.
As shown in panel 1 on the right, when RMs were inoculated with 5000 TCID50 of viral stock intracrectally the virus replicated to high titers.
Concurrent with high plasma viremia CD4+ T cells were depleted in the peripherphery as shown in Panel 2 and the GI tract as shown in Panel 3.
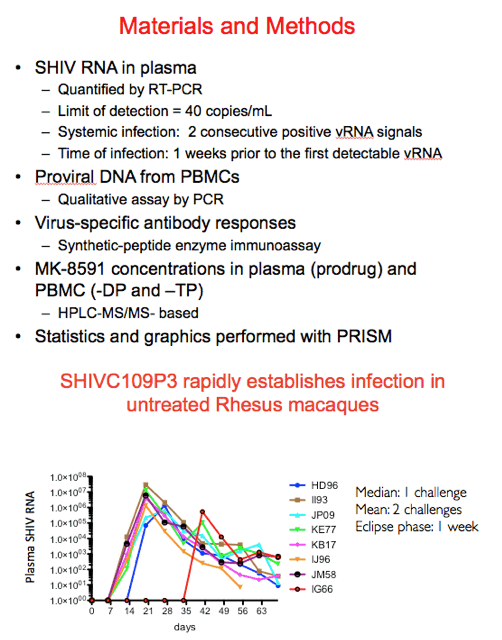
Shown in this figure are the virologic data for the control animals. 6/8 became infected after one rectal challenge, one after two and the final animal after 4. This translates into a median of 1 challenge and a mean of 2 assuming an eclipse phase of 1 week.
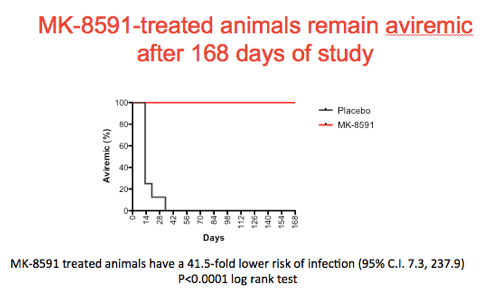
Shown is a Kaplan Meir plot summarizing the results of the experiment with the treated animals in red and the controls in black. Note that the treated animals remain aviremic through the course of the experiment.
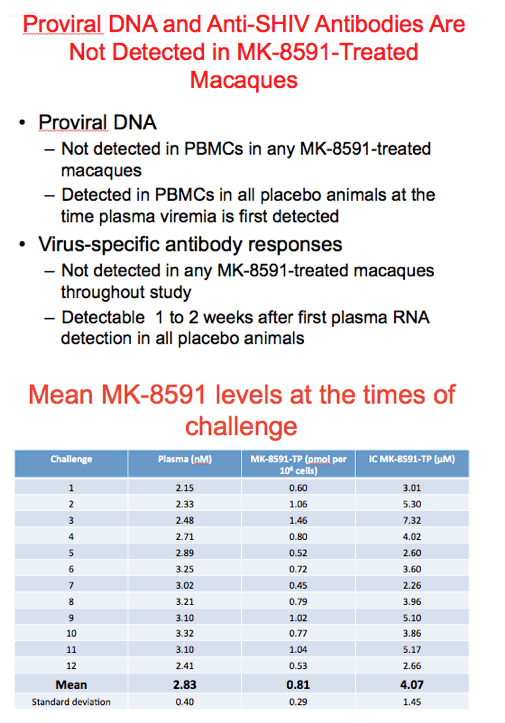
Shown in this table is a summary of the PK data at the time of each challenge. Moving from left to right are plasma concentrations of prodrug, intracellular concentration of the triphosphate expressed in pmol/106 cells and finally the intracellular concentration of the triphosphate expressed in micromolar assuming a cell volume of 200 fentoliter/cell. Note that the concentration of prodrug is predictable orders of magnitude lower that intracellular concentrations of active drug and the mean concentration of intracellular triphosphate approximates the target concentration of 1.0 pmol per 10^6 cells.

|
| |
|
 |
 |
|
|