 |
 |
 |
| |
Three Quarters of Women in HIV Group May Have ART-Hormonal Interactions
|
| |
| |
IDWeek2017/IDSA, October 4-8, 2017, San Diego
Mark Mascolini
Three quarters of women taking antiretroviral therapy (ART) and using hormonal contraception had a potential drug-drug interaction in a large Chicago clinic [1]. Among the ART-hormonal combinations used, two thirds had a potential drug-drug interaction. Two thirds of these potential interactions involved a progestin intrauterine device.
Many conception-age women with HIV rely on contraception for family planning. Safe use of hormonal contraception depends on taking an agent that does not interact with antiretrovirals. Researchers at Chicago's Northwestern University conducted this study to determine the proportion of women taking ART and using hormonal contraception at risk of a drug-drug interaction between an antiretroviral and a hormonal agent.
This retrospective chart review involved antiretroviral-treated women from 18 to 60 years old seen at the Northwestern Infectious Disease Center between January 2010 and April 2014. The researchers used electronic medical records to collect data on demographics, antiretroviral therapy, contraceptive use, and pregnancy history. They used the University of Liverpool drug-interaction guide (www.hiv-druginteractions.org) to identify potential antiretroviral-hormonal contraceptive interactions and to rank them as "potential interaction" or "do not coadminister."
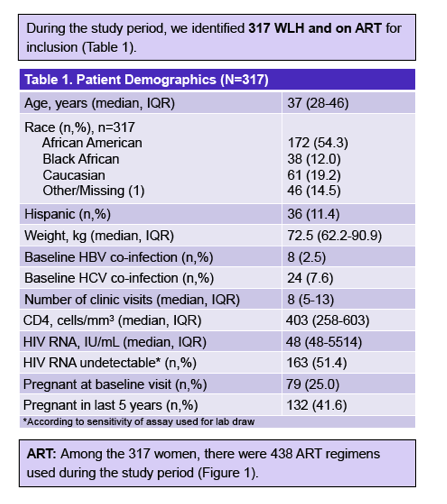
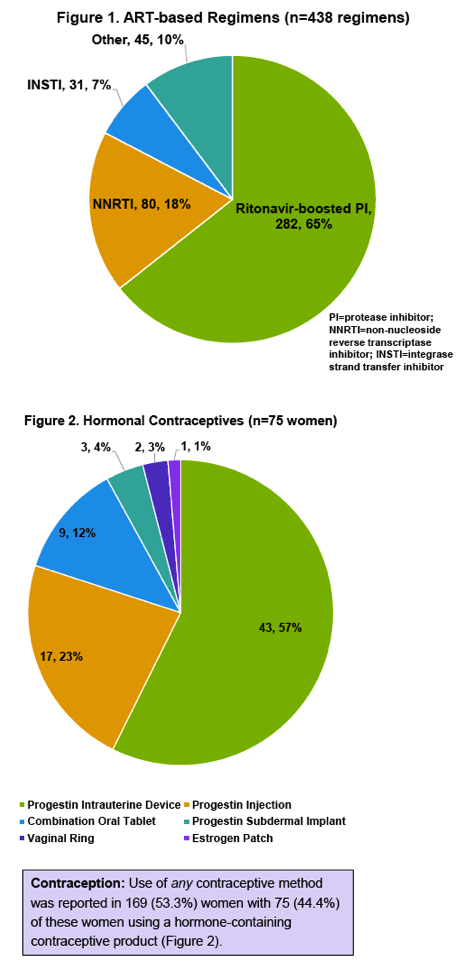
The analysis involved 317 women with a median age of 37 years (interquartile range [IQR] 28 to 46), 54% of them African American, 12% black African, 19% Caucasian, and 11% Hispanic. During the study period these women made a median of 8 clinic visits (IQR 5 to 13). About half of the women (51%) had an undetectable viral load, median CD4 count was 403 (IQR 258 to 603), and 42% of women were pregnant in the last 5 years. Women took 438 antiretroviral regimens during the study period, 65% involving a ritonavir-boosted protease inhibitor, 18% a nonnucleoside, and 7% an integrase inhibitor.
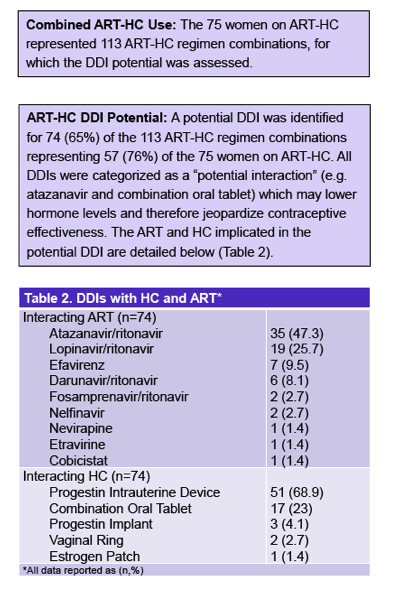
Seventy-five women (24% of 317) took a hormonal contraceptive, including 57% who used a progestin intrauterine device, 23% who used progestin injection, and 12% who used a combination oral tablet. The other women used a progestin subdermal implant, a vaginal ring, or an estrogen patch. Among the 75 women taking antiretrovirals and using a hormonal contraceptive, there were 113 antiretroviral-hormonal combinations. Seventy-four of these combinations (65%) had a potential drug-drug interaction affecting 57 of the 75 women (76%) taking antiretrovirals and using a hormonal agent. The researchers categorized all of these as "potential interactions" and none as "do not coadminister."
Among the 74 potential drug-drug interactions, the highest proportion (47%) involved atazanavir/ritonavir, while fewer involved lopinavir/ritonavir (26%), efavirenz (9.5%), or darunavir/ritonavir (8%). Only 1.4% involved cobicistat, and none involved an integrase inhibitor. The most frequent interacting hormonal contraceptives were the progestin intrauterine device (69%) and the combination oral tablet (23%), distantly followed by the progestin implant (4%), the vaginal ring (3%), and the estrogen patch (1.4%).
In summary, one quarter of antiretroviral-treated women in this urban US clinic took a hormonal contraceptive, and three quarters of the women taking ART and a hormonal agent had a potential antiretroviral-hormonal interaction. The Northwestern team suggested that future research consider the number of pregnancies that occur among HIV-positive women using contraception and "the specific role of potential drug-drug interactions in these pregnancies."
Reference
1. McLaughlin SM, Jensen A, Cohn S, Darin K. Prevalence of drug-drug interactions with hormonal contraceptives and antiretrovirals in women living with HIV. IDWeek2017/IDSA. October 4-8, 2017. San Diego. Abstract 2238.

|
| |
|
 |
 |
|
|