 |
 |
 |
| |
Incidence of symptomatic CSF viral escape in HIV infected adults receiving atazanavir/ritonavir (ATV/r)-containing ART: A tertiary care cohort in western India
|
| |
| |
Download the PDF here
Reported by Jules Levin
IDWeek2017/IDSA, October 4-8, 2017, San Diego
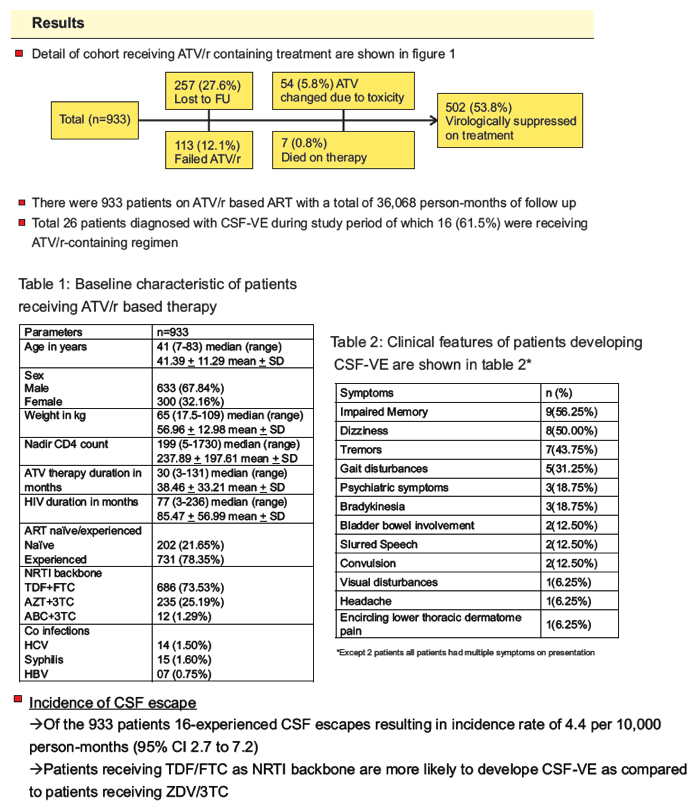
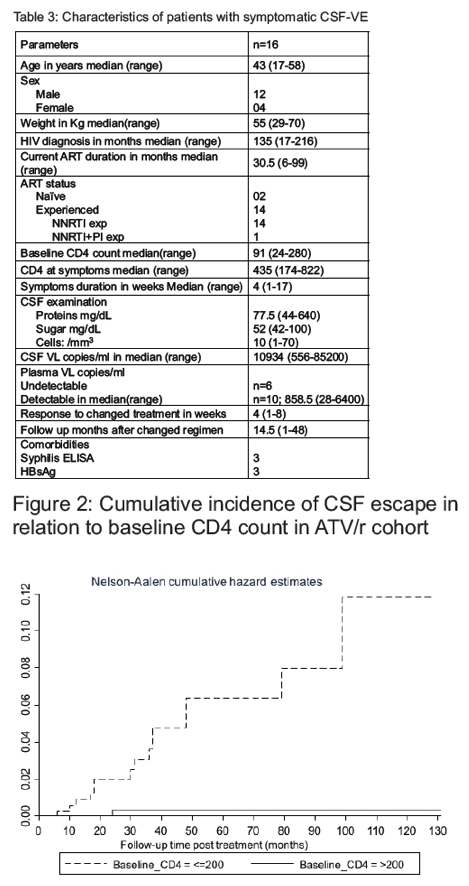
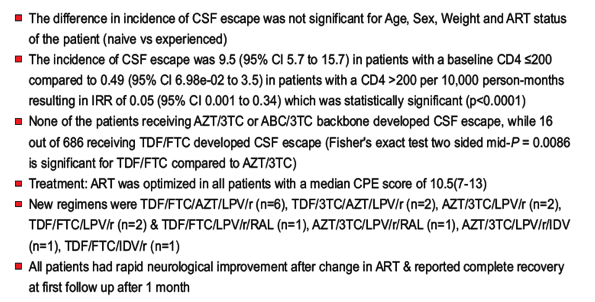
-- The incidence of CSF escape was 9.5 (95% CI 5.7 to 15.7) in patients with a baseline CD4 ≤200
compared to 0.49 (95% CI 6.98e-02 to 3.5) in patients with a CD4 >200 per 10,000 person-months
resulting in IRR of 0.05 (95% CI 0.001 to 0.34) which was statistically significant (p<0.0001)
-- None of the patients receiving AZT/3TC or ABC/3TC backbone developed CSF escape, while 16
out of 686 receiving TDF/FTC developed CSF escape (Fisher's exact test two sided mid-P = 0.0086
is significant for TDF/FTC compared to AZT/3TC)
-- Treatment: ART was optimized in all patients with a median CPE score of 10.5(7-13)
-- New regimens were TDF/FTC/AZT/LPV/r (n=6), TDF/3TC/AZT/LPV/r (n=2), AZT/3TC/LPV/r (n=2),
TDF/FTC/LPV/r (n=2) & TDF/FTC/LPV/r/RAL (n=1), AZT/3TC/LPV/r/RAL (n=1), AZT/3TC/LPV/r/IDV
(n=1), TDF/FTC/IDV/r (n=1)
-- All patients had rapid neurological improvement after change in ART & reported complete recovery
at first follow up after 1 month
Program Abstract
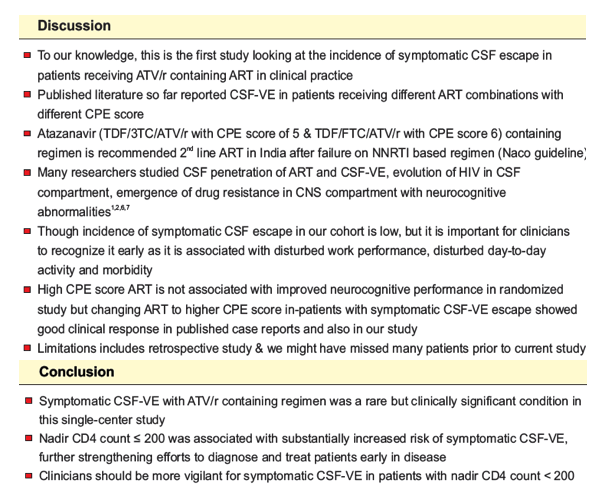
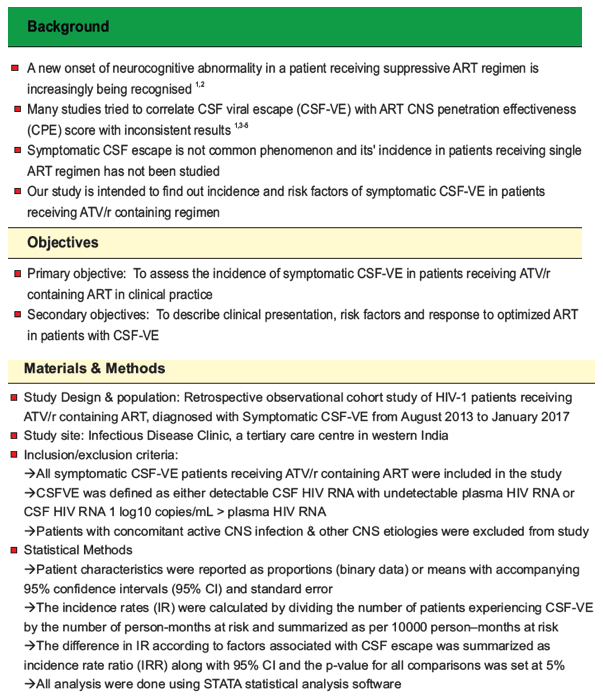
Background: CSF viral escape (CSF-VE) in patients receiving effective antiretroviral treatment (ART) has been increasingly described in the last decade. This single-center study attempts to quantify the incidence of symptomatic CSF escape in patients receiving ATV/r containing regimen.
Objectives: Primary objective was to assess the incidence of symptomatic CSF-VE in patients receiving ATV/r-containing ART in clinical practice. Secondary objectives were to describe clinical presentation, risk factors and clinical response after ART was changed.
Methods: We performed a retrospective analysis of adults receiving ATV/r-containing ART who were diagnosed with symptomatic CSF-VE from August 2013 to January 2017 at a tertiary care center. Patients with active CNS infections were excluded. Incidence rates were calculated by dividing the number of patients who experienced CSF-VE by the number of person-months at risk and summarized as per 10000 (ten thousand) person-months at risk. Difference in incidence of CSF-VE as per the ART regimen was assessed using Fisher exact test.
Results: 933 HIV-1 adults with a total of 36,068 person-months of follow up were included. Of 26 patients diagnosed with CSF-VE, 16 (61.5%) received ATV/r-containing regimens. Impaired memory (56.3%), dizziness (50%) and tremors (43.8%) were the three most common presenting symptoms. Incidence rate of symptomatic CSF-VE was 4.4 per 10,000 person-months (95% CI 2.7 to 7.2). Incidence of CSF-VE was not associated with age, sex, weight, or ART status (naïve vs first line failure) of the patient. The incidence of CSF-VE was 9.5 per 10,000 person-months (95% CI 5.7 to 15.7) when the nadir CD4 count was ²200 compared to 0.49 (95% CI 0.07 to 3.5) with a nadir CD4 count >200 (IRR 19.1 (95% CI 2.93 to 802.8), p<0.0001) (figure 1). None receiving AZT/3TC developed CSF-VE, while 16 out of 686 receiving TDF/FTC developed CSF-VE (p=0.001). ART was optimized in all patients with a median CPE score of 10.5(7-13). All patients had rapid neurological improvement after change in ART.
Conclusion: Symptomatic CSF-VE with ATV/r containing regimen was a rare but clinically significant condition in this single-center study. Nadir CD4 count ² 200 was associated with substantially increased risk of symptomatic CSF-VE, further strengthening efforts to diagnose and treat patients early in disease.

Atul Patel MD, FIDSA1,2, Ketan Patel MD2, Swati Gohel MD2, A.Kumar MD, MPH 1,Scott Letendre MD3
1Departement of Internal Medicine, University of South Florida, Tampa, FL, USA; 2Vedanta Institute of medical sciences, Infectious disease clinic, Ahmedabad- 380009, INDIA, 3Department of medicine, University of California, San Diego, LaJolla, CA, USA.
REFERENCES
1. Canestri A, Lescure FX, Jaureguiberry S, et al. Discordance between cerebral spinal fluid and plasma HIV replication in patients with neurological symptoms who are receiving suppressive antiretroviral therapy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2010;50:773-8.
2. Tamarit Mdel P, Quereda C, Gonzalez-Rozas M, Corral I, Casado JL. HIV type 1 viral encephalitis after development of viral resistance to plasma suppressive antiretroviral therapy. AIDS research and human retroviruses 2012;28:83-6.
3. Ellis RJ, Letendre S, Vaida F, et al. Randomized trial of central nervous system-targeted antiretrovirals for HIV-associated neurocognitive disorder. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 2014;58:1015-22.
4. Peluso MJ, Ferretti F, Peterson J, et al. Cerebrospinal fluid HIV escape associated with progressive neurologic dysfunction in patients on antiretroviral therapy with well controlled plasma viral load. Aids 2012;26:1765-74.
5. Fabbiani M, Grima P, Milanini B, et al. Antiretroviral neuropenetration scores better correlate with cognitive performance of HIV-infected patients after accounting for drug susceptibility. Antiviral therapy 2015;20:441-7.
6. Beguelin C, Vazquez M, Bertschi M, et al. Viral Escape in the Central Nervous System with Multidrug-Resistant Human Immunodeficiency Virus-1. Open forum infectious diseases 2016;3:ofv210.
7. Bogoch, II, Davis BT, Venna N. Reversible dementia in a patient with central nervous system escape of human immunodeficiency virus. The Journal of infection 2011;63:236-9.
|
| |
|
 |
 |
|
|