 |
 |
 |
| |
Limited sustained response and lack of HBsAg decline
after stopping long-term nucleos(t)ide analogue therapy
in HBeAg negative patients with chronic hepatitis B:
Results of the prospective, randomized, open-label phase IV
STOP study
|
| |
| |
Reported by Jules Levin
AASLD 2018 Nov 9-13 SF
Kin Seng LiemŽ1,2, Scott Fung1, David K. Wong1, Colina Yim1, Seham Noureldin1, Feng Fei Huang1, Hemant Shah1, Jordan J. Feld1,3, Bettina E. Hansen1,2,4, Harry L.A. Janssen1
1 Toronto Centre for Liver Disease, Toronto General Hospital, University Health Network, Toronto, Canada.
2 Department of Gastroenterology and Hepatology, Erasmus University Medical Center Rotterdam, Rotterdam, the Netherlands.
3 McLaughlin-Rotman Centre for Global Health, Toronto, Canada.
4 Institute of Health Policy, Management and Evaluation, University of Toronto, Toronto, Canada
program abstract
Background: Current guidelines recommend indefinite Nucleot(s)ide Analogue (NA) therapy for patients chronically infected with HBV, based on the high relapse rates observed following discontinuation of short-term therapy. However, sustained virological response (SVR) has recently been described in ~50% of patients after discontinuation of long-term NA therapy. The HBV-STOP study is a prospective multi-centre parallel cohort study of NA discontinuation in patients who have achieved long-term virological suppression on treatment. The study describes clinical outcomes post-treatment discontinuation, with the aim of identifying determinants of SVR.
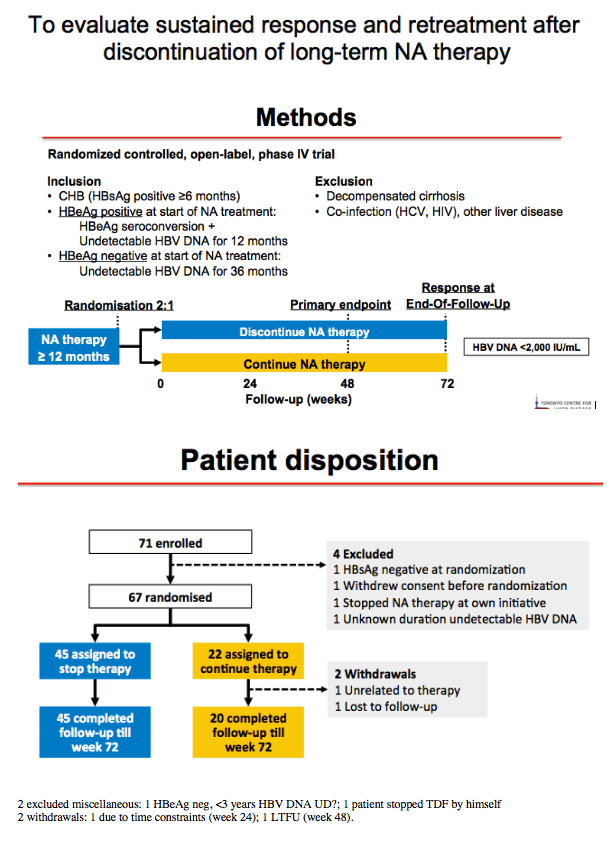
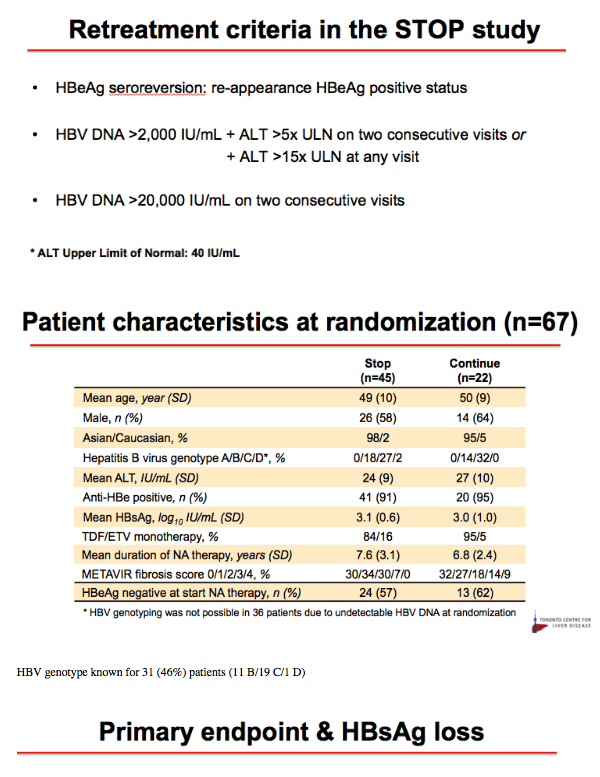
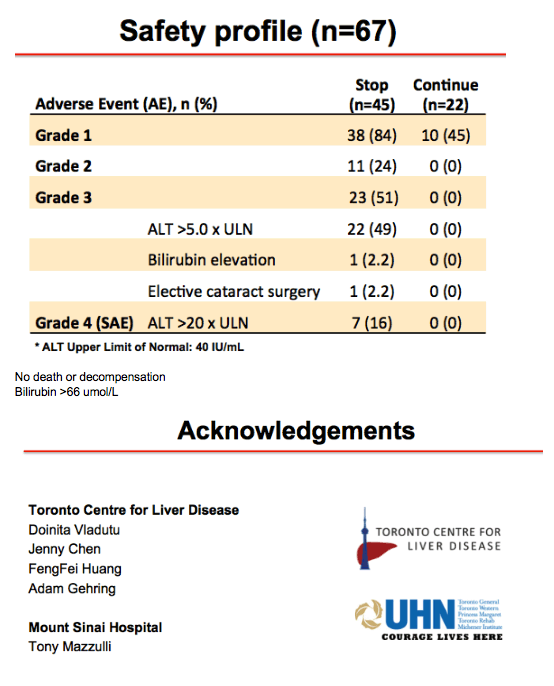
Methods: A narrative analysis of the first 44 participants recruited to 48 weeks observation was undertaken. Inclusion criteria for the study were HBeAg-negative, non-cirrhotic (Fibroscan ≤ 9.6kPa or Metavir F0-F3), virological suppression for ≥ 18 months on NA therapy uninterrupted for ≥ 2 years. All patients are being followed for 2 years. Criteria for recommencing NA therapy were HBV DNA > 2000IU/mL with either ALT > 5 x ULN for ≥ 16 weeks or ALT > 10 x ULN for ≥ 8 weeks, INR ≥ 1.5, Bilirubin > 2 x ULN, ascites, hepatic encephalopathy and investigator discretion. ULN was defined as the ULN at the site's laboratory. In this analysis, we have evaluated clinical outcomes to 48 weeks for the cohort to date, with a focus on HBV DNA suppression, ALT flare, HBsAg decline/loss, safety and need for restarting NA therapy.
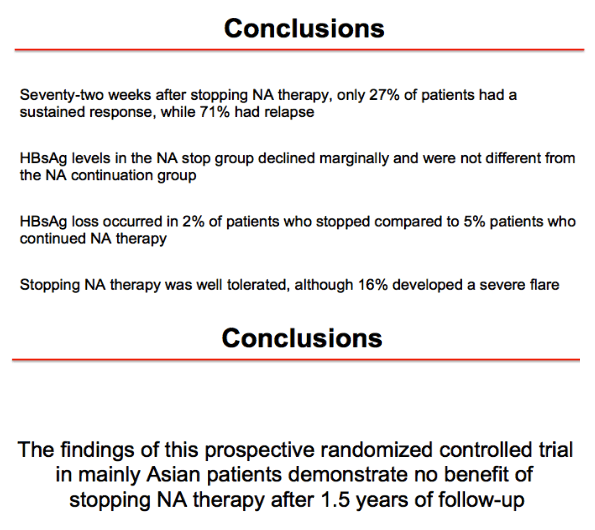
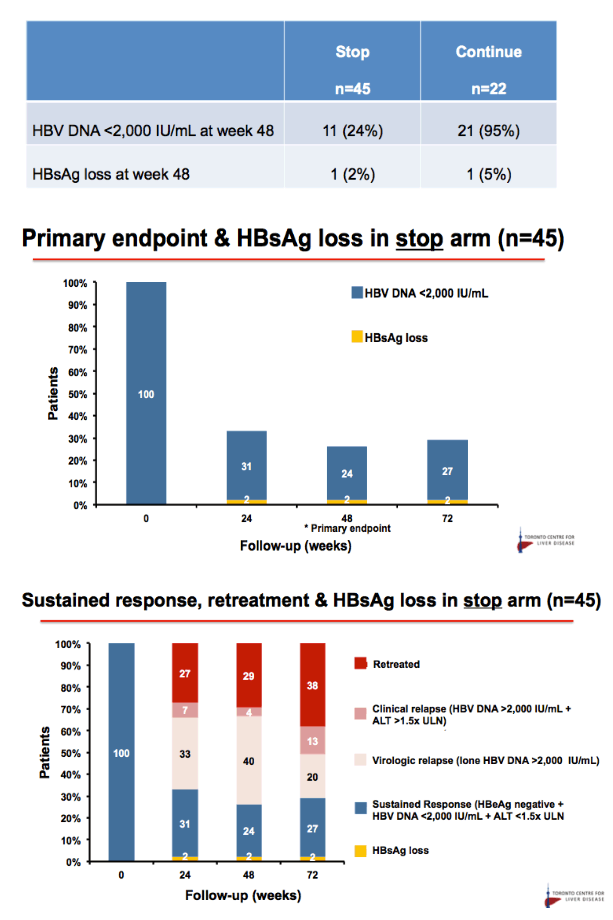
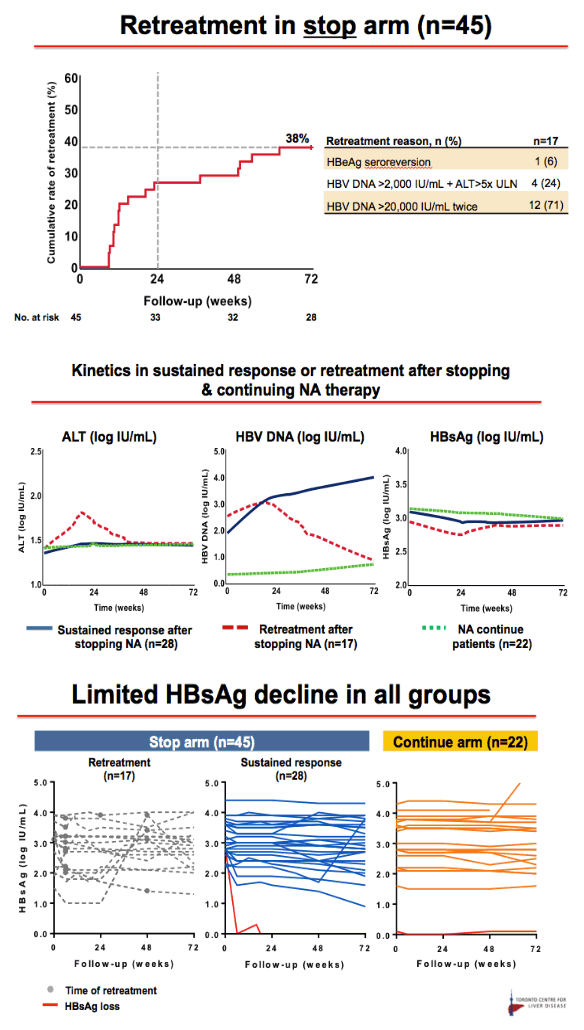
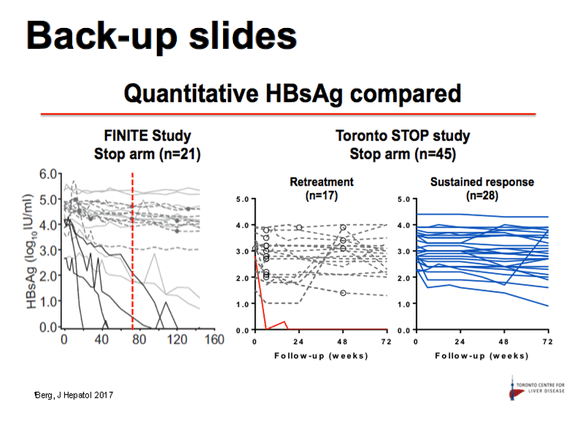
Results: Baseline characteristics included mean age of 55 years, 71% were male, and 89% were Asian. Median HBsAg level was 2135 IU/mL. All patients were non-cirrhotic. All patients experienced virological reactivation after stopping NA. At week 48, 24 (55%) are in the immune control phase (Table 1). One (2%) patient has experienced HBsAg loss, 25 (57%) have achieved > 1 log10 reduction in HBsAg level and 14 (32%) have experienced an ALT flare > 5 x ULN. Six (14%) patients have restarted NA therapy. No episodes of clinical decompensation have occurred, although bilirubin (BR) > 2 x ULN was observed in 3 patients in the context of a hepatitis flare - LFTs normalised on restarting NA therapy. Recruitment is continuing.
Conclusion: Stopping NA therapy is associated with reactivation of HBV DNA but at week 48 the majority of patients are in the immune control phase and only 14% have restarted NA therapy. HBsAg reduction >1 log was observed in 57% at 48 weeks. The study is ongoing.
|
| |
|
 |
 |
|
|