 |
 |
 |
| |
LOW DOSE MK-8591 PROTECTS RHESUS MACAQUES AGAINST RECTAL SHIV INFECTION
|
| |
| |
Reported by Jules Levin
CROI 2018 March 4-7, Boston MA
New Long Acting Potent Nuke MK-8591 + Doravirine Phase 2B Study Starts - (09/27/17)
New: MK-8591 With Doravirine and Lamivudine in Participants Infected With Human Immunodeficiency Virus Type 1 (MK-8591-011)

Program Abstract
89LB LOW DOSE MK-8591 PROTECTS RHESUS MACAQUES AGAINST RECTAL SHIV INFECTION
Martin Markowitz1, Agegnehu Gettie1, Leslie St. Bernard1, Hiroshi Mohri1, Brooke Grasperge2, James Blanchard2, Li Sun3, Kerry Fillgrove4, Daria Hazuda4, Jay Grobler4
1Aaron Diamond AIDS Research Center, New York, NY, USA, 2Tulane National Primate Research Center, Covington, LA, USA, 3Merck & Co, Inc, Upper Gwynedd, PA, USA, 4Merck & Co, Inc, West Point, PA, USA
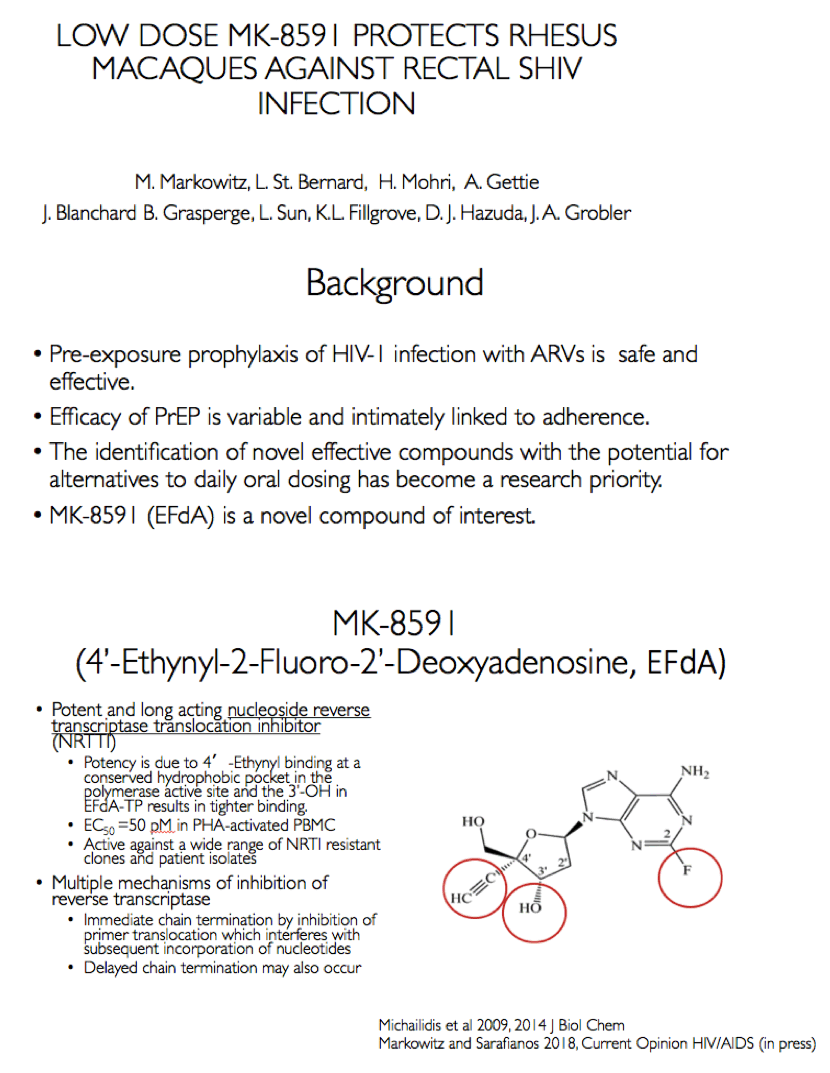
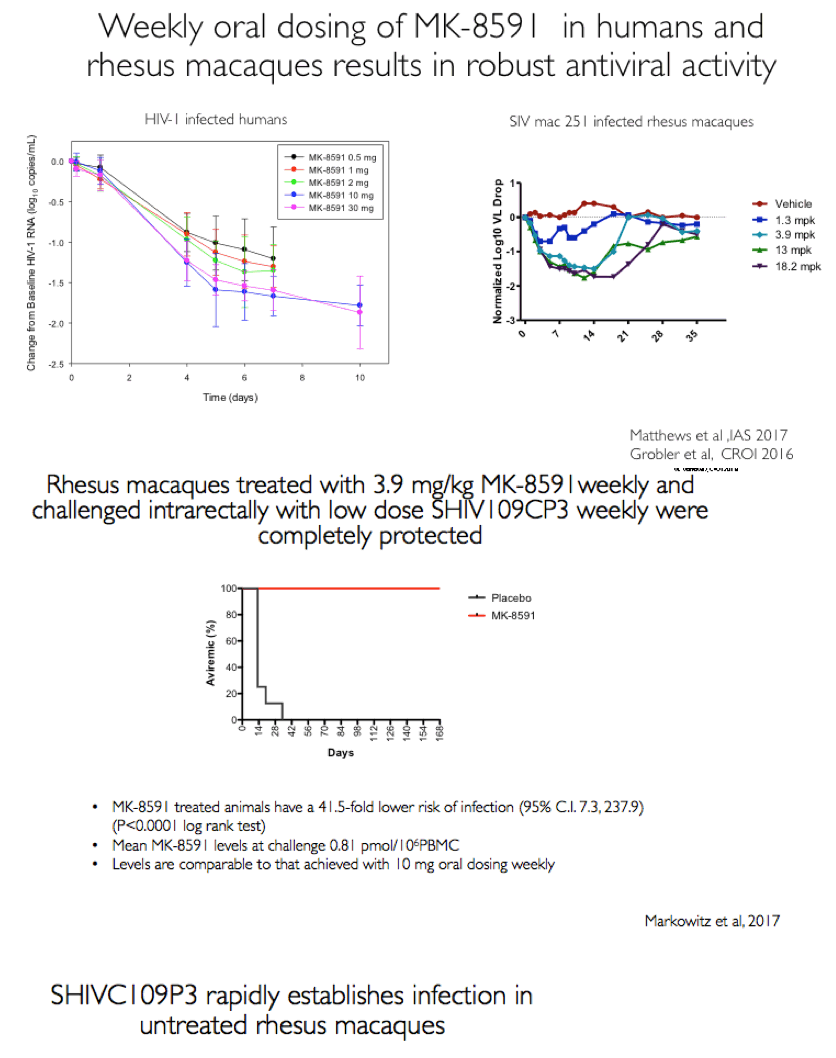
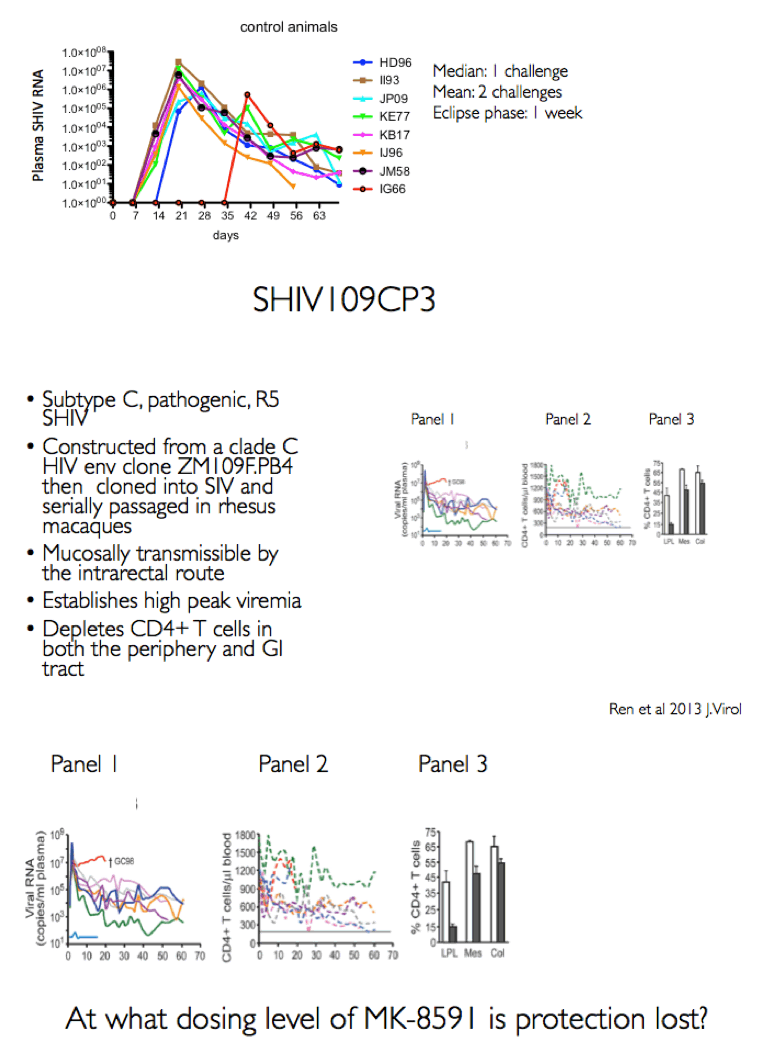
Background: MK-8591 (4'-ethynyl-2-fluoro-deoxyadenosine; EFdA), a nucleoside reverse transcriptase translocation inhibitor (NRTTI), was previously shown to completely protect rhesus macaques (RM) against SHIV infection following intrarectal (IR) challenge when dosed at 3.9 mg/kg weekly. At this dose, the mean intracellular MK-8591-triphosphate (TP) concentration of 805 fmol/10Λ6 PBMCs at time of challenge exceeds the lowest MK-8591-TP C168hr that demonstrated potent antiviral activity in HIV infected patients. To determine the lowest drug levels that confer protection, RMs were challenged at progressively lower doses until protection was no longer observed.
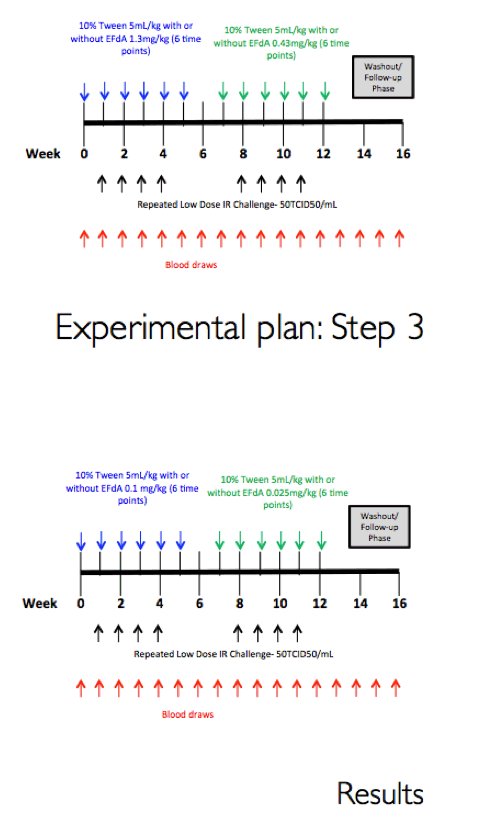
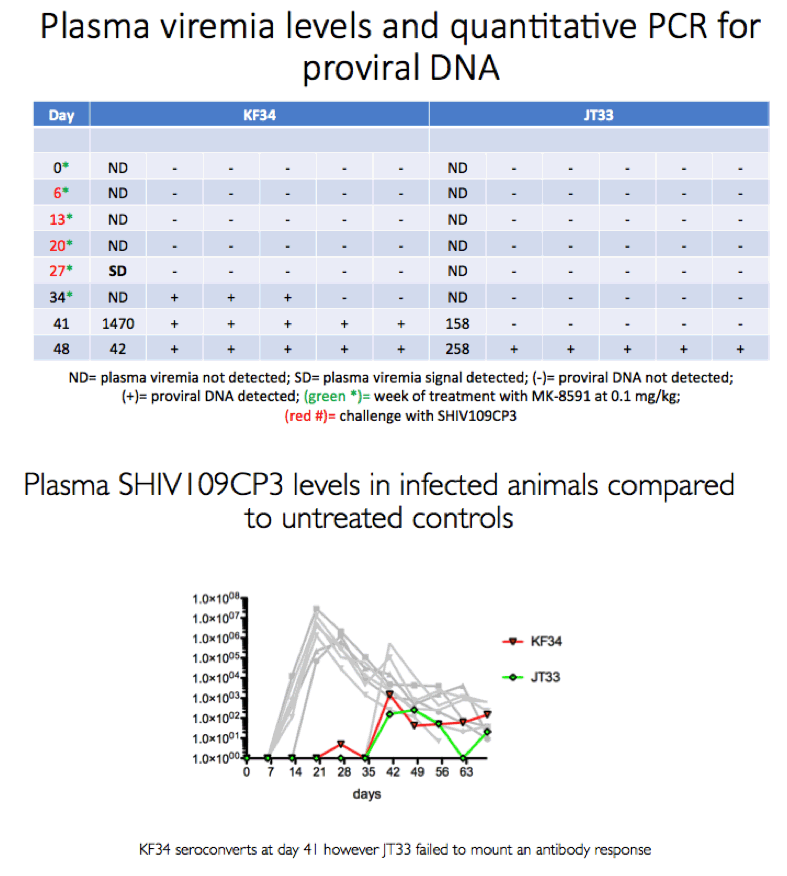
Methods: The eight male RM that had been successfully treated with 3.9 mg/kg MK-8591 after 20 weeks (w) were dosed with 1.3 mg/kg MK-8591 orally on day 0 and qw for 6 doses. All animals were again challenged IR with 1 mL of 50TCID50 of SHIVC109P3 on day 6 and weekly thereafter for up to 4 challenges or until infection was confirmed. Prior to challenge, blood was drawn for virology and PK. Infection was confirmed by real-time RT PCR in plasma. Proviral DNA was measured qw by PCR and virus-specific antibody responses were assessed. Intracellular levels of MK-8591- TP were measured by LC/MS/MS. After a 4 w washout, the treatment sequence was repeated on uninfected RM, (0.43 mg/kg weekly for 6 doses) with challenge on day 6 and weekly for up to 4 challenges or until infection was confirmed. After 10 w, this was repeated on the remaining uninfected RM at a dose of 0.1 mg/kg.
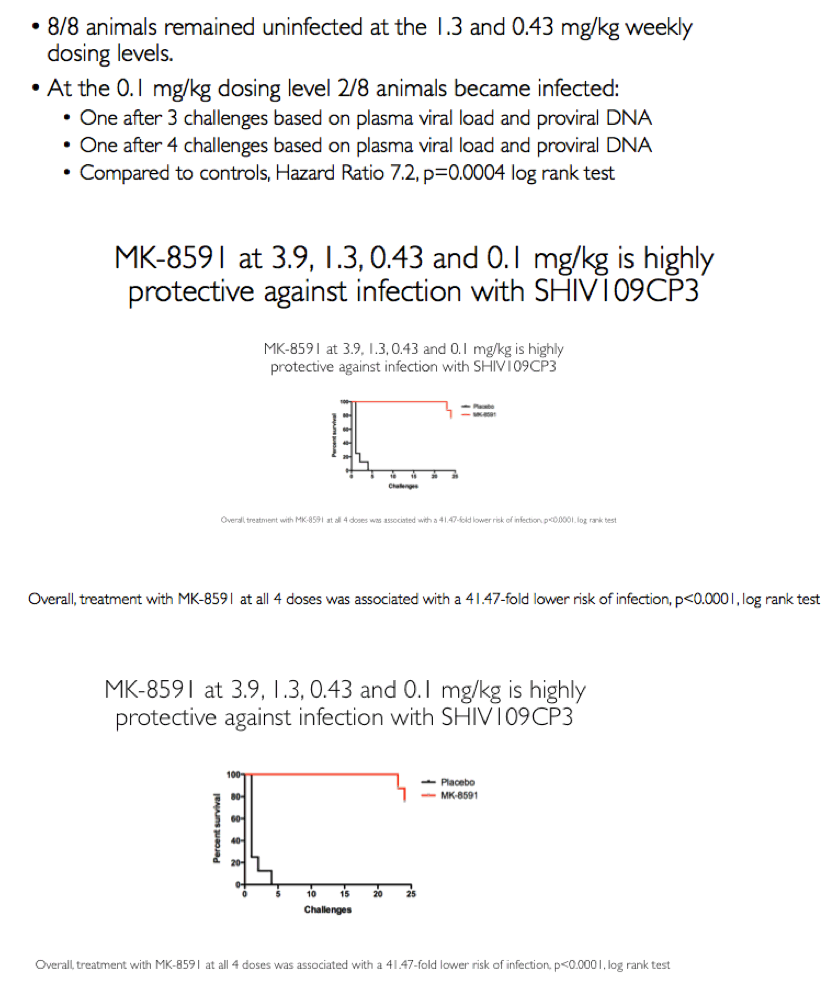
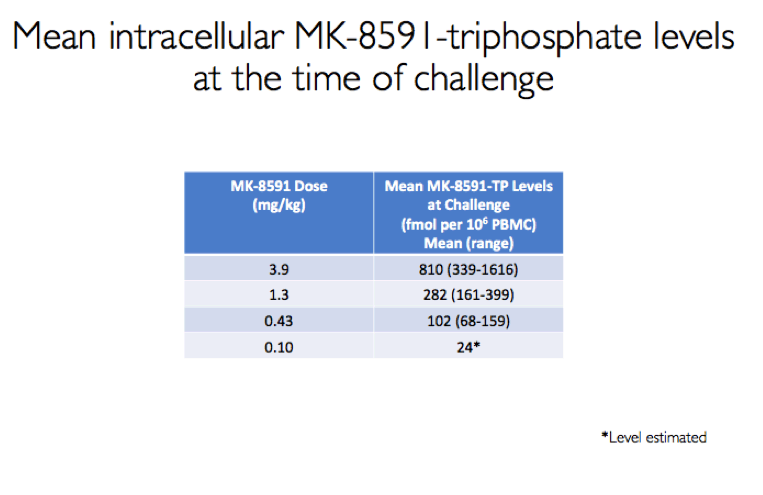
Results: All 8 animals remained uninfected after challenges at the 1.3 & 0.43 mg/kg dosing levels. At 0.1 mg/kg, 2/8 animals became infected, with one animal infected after 3 challenges and the other after 4 challenges. Mean levels of intracellular MK-8591-TP at the time of challenge were 282 and 102 fmol/10Λ6 PBMCs at the 1.3 and 0.43 mg/kg dosing levels, respectively. At the 0.1 mg/kg dose MK-8591-TP levels are estimated to be ~24 fmol/10Λ6 PBMCs.
Conclusion: In rhesus macaques MK-8591 completely protects against SHIV infection at weekly doses of 1.3 and 0.43 mg/kg and is partially protective at 0.1 mg/kg (HR 7.2, p=0.0004). MK-8591-TP levels that are protective in this model are achievable in humans at weekly doses of less than 250 μg weekly or 10 μmg daily, suggesting MK-8591 utility in extended duration prophylaxis against HIV infection.

|
| |
|
 |
 |
|
|