 |
 |
 |
| |
Integrase Inhibitor Penetration Generally Worse in Lymphoid Tissue Than Blood Cells
|
| |
| |
25th Conference on Retroviruses and Opportunistic Infections (CROI), March 4-7, 2018, Boston
Mark Mascolini
Inhibitory quotients of three integrase inhibitors generally proved much lower in lymphoid tissue than in peripheral blood mononuclear cells (PBMCs), according to results of a 34-person study by researchers at the University of Nebraska and other centers [1]. Courtney Fletcher and colleagues called for pharmacodynamic studies of integrase inhibitors in lymphoid tissue "to determine whether the lower concentrations create conditions that allow persistent viral production."
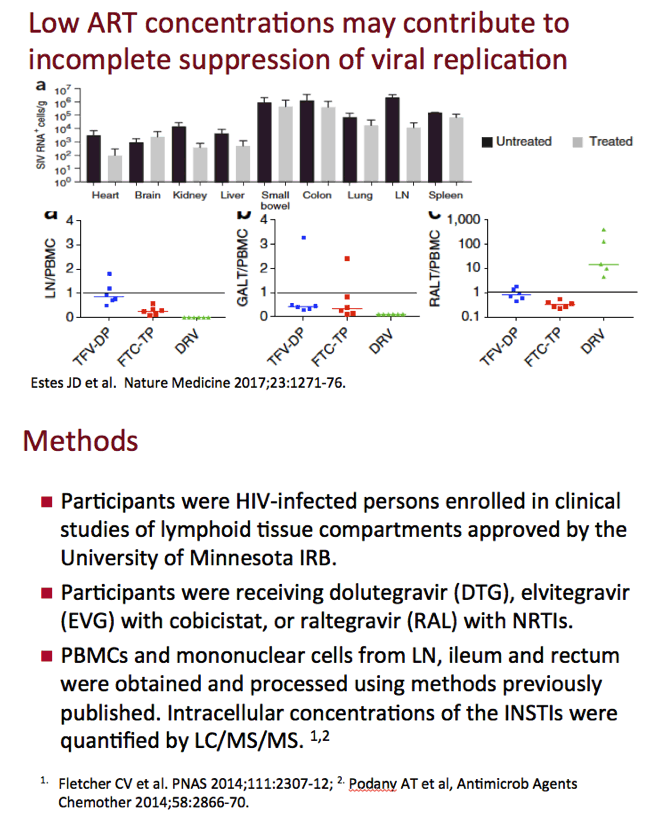
Even when viral load falls below 50 copies in plasma with antiretroviral therapy, HIV-producing cells may persist in lymph nodes and gut-associated lymphoid tissue, reservoirs that maintain the latent HIV pool. Previous work by Courtney Fletcher and colleagues showed that lymph node concentrations of antiretrovirals may be lower than levels in PBMCs and other tissues [2]. Low concentrations of antiretrovirals can favor incomplete suppression of HIV replication.
The University of Nebraska team conducted this study to assess lymphoid tissue concentrations of three licensed integrase inhibitors: dolutegravir, elvitegravir (boosted by cobicistat), and raltegravir. HIV-positive participants were enrolled in clinical lymphoid tissue studies approved by the University of Minnesota. They were taking one of the integrase inhibitors plus nucleos(t)ide reverse transcriptase inhibitors.
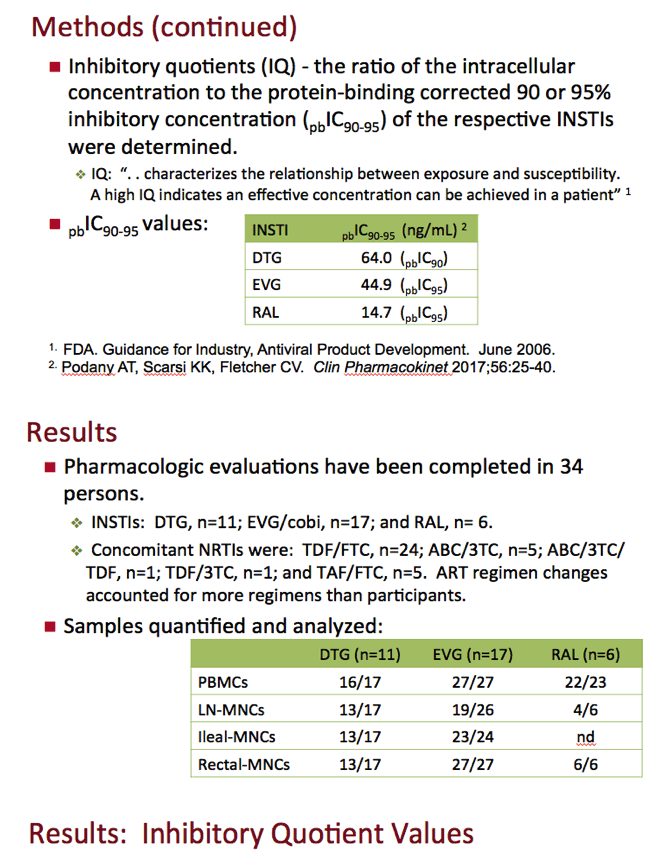
The investigators measured intracellular integrase inhibitor concentrations in samples from PBMCs and mononuclear cells from lymph nodes, ileum, and rectum. An inhibitory quotient (IQ) is the ratio of a drug's intracellular concentration to the protein binding-corrected 90% or 95% inhibitory concentration (IC90-95). In plain language, Fletcher noted, IQ means "how much drug you've got versus how much you need." IC90-95s are 64.0 ng/mL for dolutegravir, 44.9 ng/mL for elvitegravir, and 14.7 ng/mL for raltegravir [3].
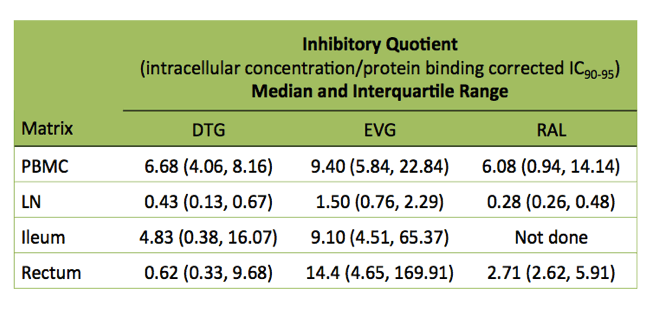
So far the researchers have completed pharmacologic evaluations in 34 people, 11 taking dolutegravir, 17 elvitegravir, and 6 raltegravir with TDF, TAF, or abacavir plus FTC or 3TC. The researchers calculated that integrase inhibitor IQs in PBMCs were 6-fold or higher than the IC90-95 for each drug. IQs in lymphoid tissues were almost always much lower than in PBMCs. The exceptions were elvitegravir IQs in ileum (median 9.10) and rectum (median 14.4) versus 9.40 in PBMCs. Median elvitegravir IQ stood at 1.50 in lymph nodes. That was the only lymph node IQ above 1.0, that is, above the IC90-95.
Median IQs for dolutegravir were 6.68 in PBMCs, 0.43 in lymph nodes, 4.83 in ileum, and 0.62 in rectum. Median values for raltegravir were 6.08 in PBMCs, 0.28 in lymph nodes, and 2.71 in rectum. (Raltegravir IQ90-95 has not been assessed in ileum.)
The researchers noted that their findings on integrase inhibitor penetration of lymphoid tissue are consistent with an in vitro model predicting the greatest lymphoid tissue penetration by elvitegravir, followed by dolutegravir then raltegravir. Standard doses of the three integrase inhibitors achieve plasma IQs of 8 or greater. But the new findings "indicate that the same pharmacokinetic conditions are not achieved" in lymphoid tissue. Fletcher added that these results confirm the long-held belief that "ability to predict tissue concentration from plasma is not very good."
References
1. Fletcher CV, Thorkelson A, Winchester L, et al. Comparative lymphoid tissue pharmacokinetics (PKs) of integrase inhibitors (INSTI). 25th Conference on Retroviruses and Opportunistic Infections (CROI). March 4-7, 2018. Boston. Abstract 27.
2. Fletcher CV, Staskus K, Wietgrefe SW, et al. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci USA. 2014;111:2307-2312. http://www.pnas.org/content/111/6/2307.long
3. Podany AT, Scarsi KK, Fletcher CV. Comparative clinical pharmacokinetics and pharmacodynamics of hiv-1 integrase strand transfer inhibitors. Clin Pharmacokinet. 2017;56:25-40.
|
| |
|
 |
 |
|
|