 |
 |
 |
| |
Week 48 Resistance Analyses of the Once-daily, Single-tablet Regimen Darunavir/Cobicistat/
Emtricitabine/Tenofovir Alafenamide (D/C/F/TAF) in Adults Living with HIV-1 from the
AMBER and EMERALD Phase 3 Trials
|
| |
| |
Download the PDF here
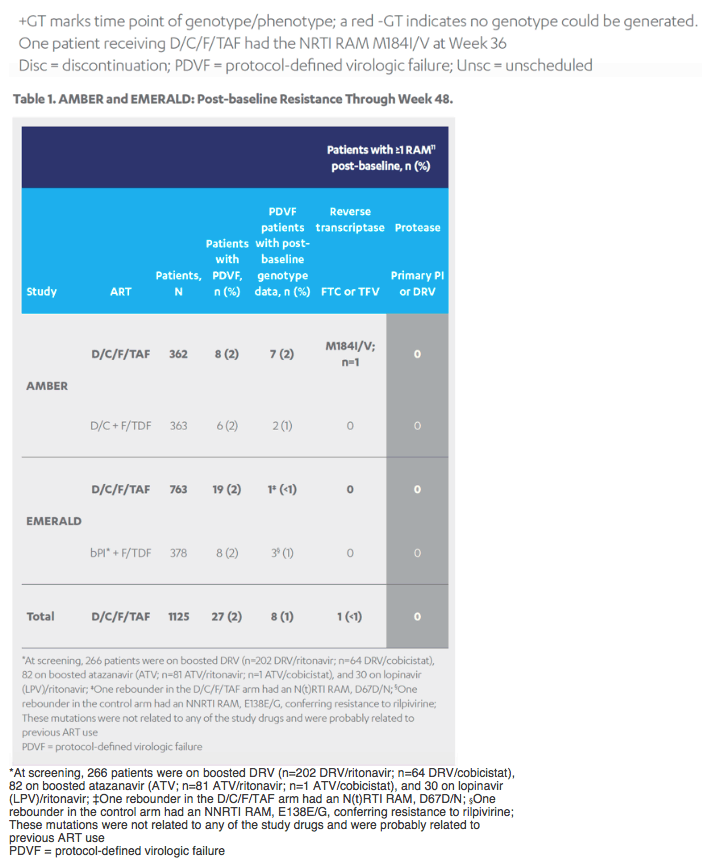
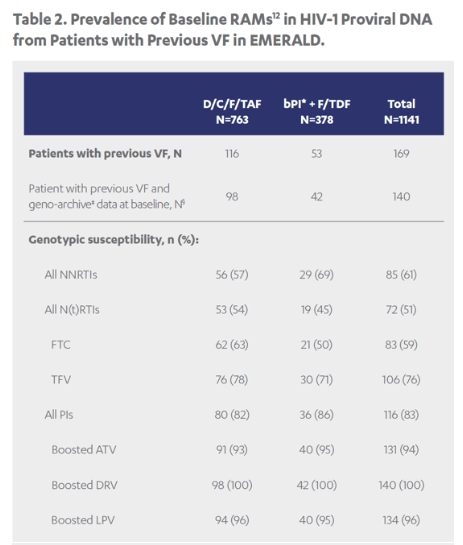
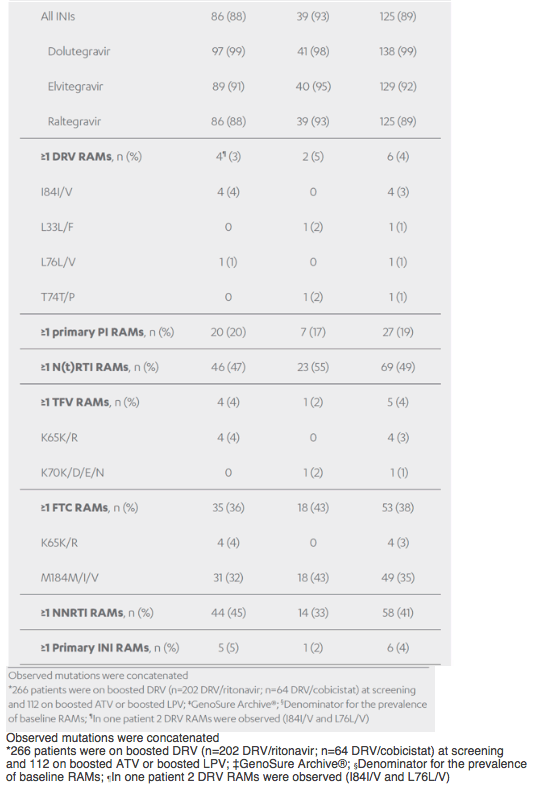
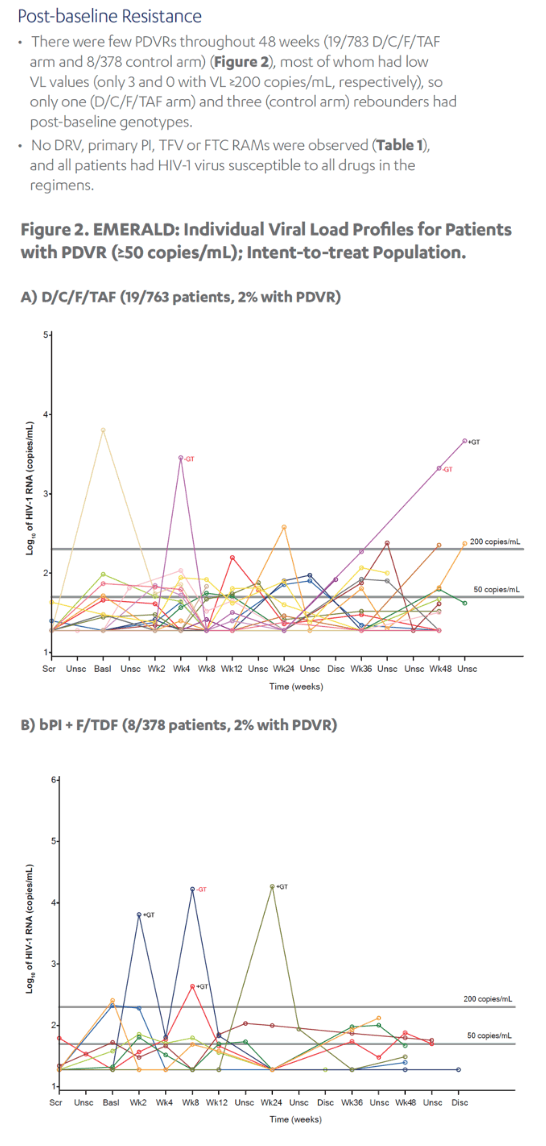
Revised poster data - wrong mutations reported previously - some reported archived mutations in EMERALD were double counted. Some mutations (eg, V32I) occur in both the protease and integrase gene, but of course only the one in protease should be counted from a resistance evaluation perspective. So after making the corrections, there are fewer subjects (only 6 with prior VF) with archived DRV RAMs and all responded (and 0 rebounders had DRV RAMs instead of 4), which means the resistance profile is cleaner than previously communicated. There were also 2 fewer subjects with archived TFV RAMs (5 instead of 7).
Reported by Jules Levin
HIV Glasgow Drug Therapy Meeting; Glasgow, UK, 28-31 October 2018
Erkki Lathouwers1, Eric Y. Wong2, Kimberley Brown1, Bryan Baugh3, Anne Ghys1, John Jezorwski4, El Ghazi Mohsine1, Erika Van Landuyt1,
Magda Opsomer1, Sandra De Meyer1
1Janssen Pharmaceutica NV, Beerse, Belgium; 2Janssen Scientific Affairs, LLC, Titusville, NJ, USA; 3Janssen Research & Development LLC, Raritan, NJ, USA;
4Janssen Research & Development, Pennington, NJ, USA





|
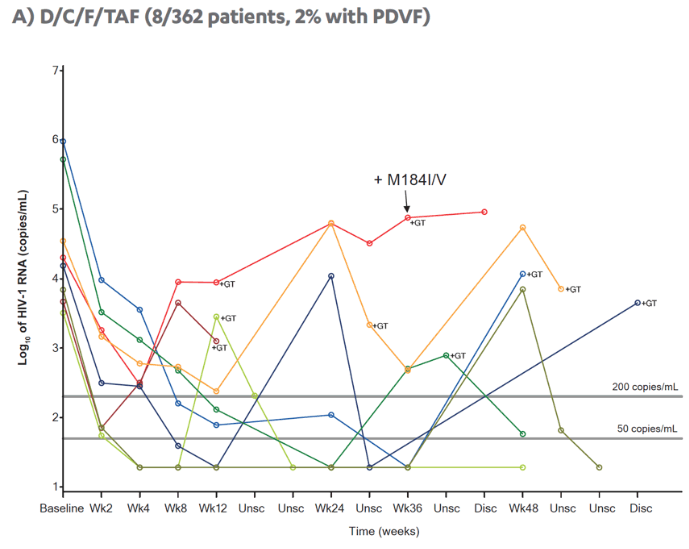
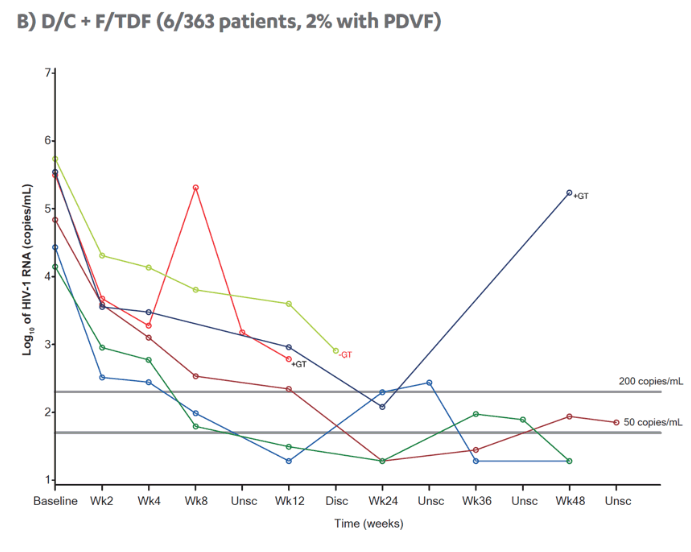
| |
|
 |
 |
|
|