| |
U.S. FDA Accepts sNDAs for PIFELTRO™ (doravirine) and DELSTRIGO™ (doravirine/lamivudine/tenofovir disoproxil fumarate)
|
| |
| |
Potential New Indication Would Expand Use to Allow Treatment-Experienced Adults Living with HIV-1 Whose Virus is Suppressed to Switch to PIFELTRO (in Combination with Other Antiretrovirals) or DELSTRIGO
Tuesday, January 22, 2019 4:30 pm EST
KENILWORTH, N.J., Jan. 22, 2019 – Merck (NYSE: MRK), known as MSD outside the United States and Canada, today announced that the U.S. Food and Drug Administration (FDA) has accepted for review supplemental New Drug Applications (sNDAs) for PIFELTRO™ and DELSTRIGO™. The applications seek approval for PIFELTRO (in combination with other antiretroviral medicines) and DELSTRIGO for use in people living with HIV-1 who are switching from a stable antiretroviral regimen and whose virus is suppressed (HIV-1 RNA <50 copies/mL). The Prescription Drug User Fee Act (PDUFA) date for the sNDAs is Sept. 20, 2019.
PIFELTRO (doravirine, 100 mg), a once-daily non-nucleoside reverse transcriptase inhibitor (NNRTI) to be administered in combination with other antiretroviral medicines, and DELSTRIGO, a once-daily fixed-dose combination tablet of doravirine (100 mg), lamivudine (3TC, 300 mg) and tenofovir disoproxil fumarate (TDF, 300 mg) as a complete regimen, are currently indicated for the treatment of HIV-1 infection in adult patients not previously treated with antiretroviral therapy. DELSTRIGO contains a boxed warning regarding post-treatment acute exacerbations of hepatitis B (HBV) infection.PIFELTRO and DELSTRIGO do not cure HIV-1 infection or AIDS.
"Our clinical development program continues to generate meaningful evidence for PIFELTRO and DELSTRIGO in people living with HIV," said Dr. Michael Robertson, executive director and section head for HIV and HCV, Merck Research Laboratories. "We are pleased that the FDA has accepted these supplemental new drug applications. We look forward to continuing our work with the goal of expanding HIV treatment options."
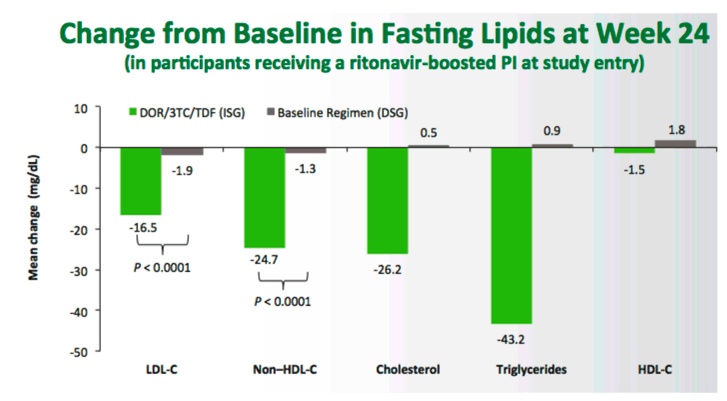
The submissions were based on the results of the Phase 3 DRIVE-SHIFT trial which met its primary endpoint, demonstrating non-inferior efficacy of switching to DELSTRIGO (doravirine/lamivudine (3TC)/tenofovir disoproxil fumarate (TDF)) compared with continuing on a baseline regimen of two nucleoside reverse transcriptase inhibitors (NRTIs) plus a boosted protease inhibitor, boosted elvitegravir, or NNRTI. Non-inferior efficacy was measured by the proportion of participants who switched to DELSTRIGO and had plasma HIV-1 RNA levels <50 copies/mL at Week 48 compared to the proportion of participants who continued on their baseline regimen and had HIV-1 RNA levels <50 copies/mL at Week 24. The resultswere presented at IDWeek 2018 in San Francisco.
It has been recruiting & is ongoing: MK-8591 With Doravirine and Lamivudine in Participants Infected With Human Immunodeficiency Virus Type 1 (MK-8591-011)
New Long Acting Potent Nuke MK-8591 + Doravirine Phase 2B Study Starts
Rational Design of Doravirine (DOR): A Review of Development From Bench to Patients
Switch to Doravirine/Lamivudine/Tenofovir Disoproxil Fumarate (DOR/3TC/TDF) Maintains Virologic Suppression Through 48 Weeks: Results of the DRIVE-SHIFT Trial
Selected Safety Information about PIFELTRO (doravirine) in the U.S.
PIFELTRO is contraindicated when co-administered with drugs that are strong cytochrome P450 (CYP)3A enzyme inducers (including the anticonvulsants carbamazepine, oxcarbazepine, phenobarbital, and phenytoin; the androgen receptor inhibitor enzalutamide; the antimycobacterials rifampin and rifapentine; the cytotoxic agent mitotane; and the herbal product St. John's wort (Hypericum perforatum)), as significant decreases in PIFELTRO plasma concentrations may occur, which may decrease the effectiveness of PIFELTRO.Immune reconstitution syndrome can occur, including the occurrence of autoimmune disorders with variable time to onset, which may necessitate further evaluation and treatment.Co-administration of PIFELTRO with efavirenz, etravirine or nevirapine is not recommended.If co-administered with rifabutin, increase PIFELTRO dosage to one tablet twice daily (approximately 12 hours apart).
Consult the full Prescribing Information prior to and during treatment for important potential drug-drug interactions.The safety of PIFELTRO is based on two studies, DRIVE-FORWARD and DRIVE-AHEAD.In DRIVE-FORWARD, the most common adverse reactions (incidence ≥5%, all intensities) were nausea (7%), headache (6%), fatigue (6%), diarrhea (5%) and abdominal pain (5%).In DRIVE-AHEAD, the most common adverse reactions (incidence ≥5%, all intensities) were dizziness (7%), abnormal dreams (5%) and nausea (5%).
There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to PIFELTRO during pregnancy.Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry at 1-800-258-4263.Mothers infected with HIV-1 should be instructed not to breastfeed if they are receiving PIFELTRO (doravirine) due to the potential for HIV-1 transmission.
Selected Safety Information about DELSTRIGO (doravirine/lamivudine (3TC)/tenofovir disoproxil fumarate (TDF)) in the U.S.
Warning: Post treatment Acute Exacerbation of Hepatitis B (HBV)
All patients with HIV-1 should be tested for the presence of HBV before initiating antiretroviral therapy.Severe acute exacerbations of HBV have been reported in patients who are coinfected with HIV-1 and HBV and have discontinued products containing lamivudine or TDF, which are components of DELSTRIGO. Patients coinfected with HIV-1 and HBV who discontinue DELSTRIGO should be monitored with both clinical and laboratory follow-up for at least several months after stopping DELSTRIGO.If appropriate, initiation of anti-HBV therapy may be warranted.
DELSTRIGO is contraindicated when co-administered with drugs that are strong cytochrome P450 (CYP)3A enzyme inducers (including the anticonvulsants carbamazepine, oxcarbazepine, phenobarbital, and phenytoin; the androgen receptor inhibitor enzalutamide; the antimycobacterials rifampin and rifapentine; the cytotoxic agent mitotane; and the herbal product St. John's wort (Hypericum perforatum)), as significant decreases in doravirine plasma concentrations may occur, which may decrease the effectiveness of DELSTRIGO. DELSTRIGO is contraindicated in patients with a previous hypersensitivity reaction to lamivudine.
Renal impairment, including cases of acute renal failure and Fanconi syndrome, have been reported with the use of TDF.DELSTRIGO should be avoided with concurrent or recent use of a nephrotoxic agent, as cases of acute renal failure after initiation of high-dose or multiple NSAIDs have been reported in patients with risk factors for renal dysfunction who appeared stable on TDF.
Prior to or when initiating DELSTRIGO, and during treatment, assess serum creatinine, estimated creatinine clearance, urine glucose and urine protein in all patients.In patients with chronic kidney disease, also assess serum phosphorus.Discontinue DELSTRIGO in patients who develop clinically significant decreases in renal function or evidence of Fanconi syndrome.Discontinue DELSTRIGO if estimated creatinine clearance declines below 50 mL/min.
In clinical trials in HIV-1 infected adults, TDF was associated with slightly greater decreases in bone mineral density (BMD) and increases in biochemical markers of bone metabolism. Serum parathyroid hormone levels and 1,25 vitamin D levels were also higher. Cases of osteomalacia associated with proximal renal tubulopathy have been reported with the use of TDF.
Immune reconstitution syndrome can occur, including the occurrence of autoimmune disorders with variable time to onset, which may necessitate further evaluation and treatment.Because DELSTRIGO (doravirine/lamivudine (3TC)/tenofovir disoproxil fumarate (TDF)) is a complete regimen, co-administration with other antiretroviral medications for the treatment of HIV-1 infection is not recommended.
Consult the full Prescribing Information prior to and during treatment for important potential drug-drug interactions.
If co-administered with rifabutin, take one tablet of DELSTRIGO once daily, followed by one tablet of doravirine (PIFELTRO) approximately 12 hours after the dose of DELSTRIGO.The most common adverse reactions with DELSTRIGO (incidence ≥5%, all intensities) were dizziness (7%), nausea (5%) and abnormal dreams (5%).
There is a pregnancy exposure registry that monitors pregnancy outcomes in individuals exposed to DELSTRIGO during pregnancy.Healthcare providers are encouraged to register patients by calling the Antiretroviral Pregnancy Registry at 1-800-258-4263.Mothers infected with HIV-1 should be instructed not to breastfeed if they are receiving DELSTRIGO due to the potential for HIV-1 transmission.Because DELSTRIGO is a fixed-dose combination tablet and the components cannot be altered, it is not recommended in patients with estimated creatinine clearance less than 50 mL/min.
About Merck
For more than a century, Merck, a leading global biopharmaceutical company known as MSD outside of the United States and Canada, has been inventing for life, bringing forward medicines and vaccines for many of the world's most challenging diseases. Through our prescription medicines, vaccines, biologic therapies and animal health products, we work with customers and operate in more than 140 countries to deliver innovative health solutions. We also demonstrate our commitment to increasing access to health care through far-reaching policies, programs and partnerships. Today, Merck continues to be at the forefront of research to advance the prevention and treatment of diseases that threaten people and communities around the world - including cancer, cardio-metabolic diseases, emerging animal diseases, Alzheimer's disease and infectious diseases including HIV and Ebola. For more information, visit www.merck.comand connect with us on Twitter, Facebook, Instagram,YouTubeand LinkedIn.
Forward-Looking Statement of Merck & Co., Inc., Kenilworth, N.J., USA
This news release of Merck & Co., Inc., Kenilworth, N.J., USA (the "company") includes "forward-looking statements" within the meaning of the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995. These statements are based upon the current beliefs and expectations of the company's management and are subject to significant risks and uncertainties. There can be no guarantees with respect to pipeline products that the products will receive the necessary regulatory approvals or that they will prove to be commercially successful. If underlying assumptions prove inaccurate or risks or uncertainties materialize, actual results may differ materially from those set forth in the forward-looking statements.
Risks and uncertainties include but are not limited to, general industry conditions and competition; general economic factors, including interest rate and currency exchange rate fluctuations; the impact of pharmaceutical industry regulation and health care legislation in the United States and internationally; global trends toward health care cost containment; technological advances, new products and patents attained by competitors; challenges inherent in new product development, including obtaining regulatory approval; the company's ability to accurately predict future market conditions; manufacturing difficulties or delays; financial instability of international economies and sovereign risk; dependence on the effectiveness of the company's patents and other protections for innovative products; and the exposure to litigation, including patent litigation, and/or regulatory actions.
The company undertakes no obligation to publicly update any forward-looking statement, whether as a result of new information, future events or otherwise. Additional factors that could cause results to differ materially from those described in the forward-looking statements can be found in the company's 2017 Annual Report on Form 10-K and the company's other filings with the Securities and Exchange Commission (SEC) available at the SEC's Internet site (www.sec.gov).
Please see Prescribing Information for DELSTRIGO (doravirine/3TC/TDF) at:
https://www.merck.com/product/usa/pi_circulars/d/delstrigo/delstrigo_pi.pdf and Patient Information for
DELSTRIGO (doravirine/3TC/TDF) at:
https://www.merck.com/product/usa/pi_circulars/d/delstrigo/delstrigo_ppi.pdf
Please see Prescribing Information for PIFELTRO (doravirine) at: https://www.merck.com/product/usa/pi_circulars/p/pifeltro/pifeltro_pi.pdf
and Patient Information for PIFELTRO (doravirine) at:
https://www.merck.com/product/usa/pi_circulars/p/pifeltro/pifeltro_ppi.pdf
Media:
Pamela Eisele
(267) 305-3558
Sarra S. Herzog
(908) 740-1871
Investors:
Teri Loxam
(908) 740-1986
Michael DeCarbo
(908) 740-1807
|
|
| |
| |
|
|
|