| |
PrEP
|
| |
| |
IAS: Changes in Truvada® for HIV Pre-exposure Prophylaxis Utilization in the USA: 2012-2016 - Disparities - (08/02/17)
IAS: Reasons for not using PrEP in a national on-line sample of U.S. men who have sex with men (MSM) - Cost, Side Effects, Access Lead Reasons for PrEP Nonuse by US MSM - (07/31/17)
IAS: Limited implementation of HIV pre-exposure prophylaxis among public health departments in North Carolina, United States - (08/02/17)
Long acting injectable Cabotegravir for PrEP is in Phase 3 [every 4 or 8 weeks] - (09/09/17)
Long-Acting HIV PrEP & Treatment for Women & Men / Once A Month or 8 Weeks Injection - (10/02/17)
Experiences with long acting injectable ART: A qualitative study among PLHIV participating in a Phase II study of cabotegravir + rilpivirine (LATTE-2) in the United States and Spain - (01/17/18)
Brief Report: The Right People, Right Places, and Right Practices Disparities in PrEP Access Among African American Men, Women, and MSM in the Deep South - (01/17/18)
Evolving Models and Ongoing Challenges for HIV Preexposure Prophylaxis Implementation in the United States - (01/17/18)
Partnerships Between a University-Affiliated Clinic and Community-Based Organizations to Reach Black Men Who Have Sex With Men for PrEP Care - (01/17/18) Duke PrEP Clinic
Changes in Kidney Function Associated With Daily Tenofovir Disoproxil Fumarate/Emtricitabine for HIV Preexposure Prophylaxis Use in the United States Demonstration Project - (01/17/18)
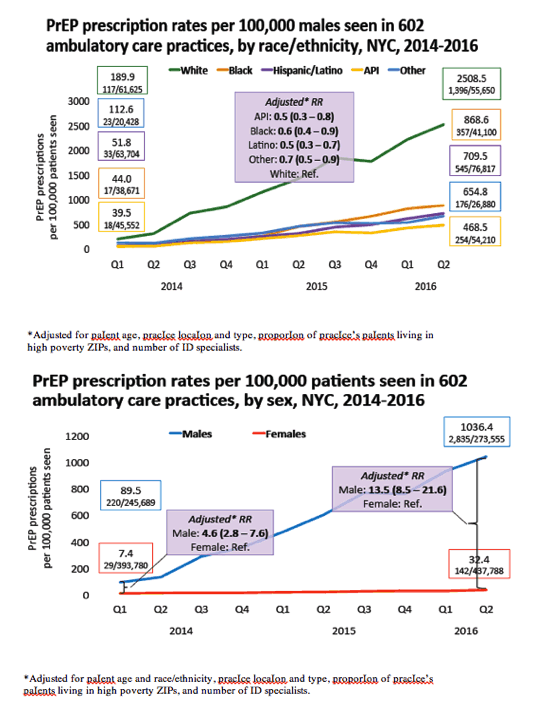
Trends and Associations with PrEP Prescription among 602 NYC Ambulatory Care Practices, 2014-16...... "PrEP prescriptIon increased 976% between Q1 2014 to Q2 2016 among 602 ambulatory care practIces in NYC" - (10/11/17)
"In New York, roughly 30 percent of gay and bisexual men are using Truvada now, up dramatically from a few years ago, according to Dr. Demetre Daskalakis, a deputy commissioner of the city's health department.
However, Daskalakis said use among young black and Hispanic men - who account for a majority of new HIV diagnoses - lags behind. To address that, the city is making Truvada readily available in some clinics in or near heavily black and Hispanic neighborhoods.
"We like to go to the root of the problem," said Daskalakis, who personally posed for the "Bare It All" campaign."Speaking to Daily Mail Online, Demetre Daskalakis, head of the HIV division at New York City's Department of Health, said the news is an exciting breakthrough, but conceded that more needs to be done to broaden coverage for women and ethnic minorities."
Usage remains low for pill that can prevent HIV infection
By Associated Press
January 8, 2018
EW YORK - From gritty neighborhoods in New York and Los Angeles to clinics in Kenya and Brazil, health workers are trying to popularize a pill that has proven highly effective in preventing HIV but which - in their view - remains woefully underused.
Marketed in the United States as Truvada, and sometimes available abroad in generic versions, the pill has been shown to reduce the risk of getting HIV from sex by more than 90 percent if taken daily. Yet worldwide, only about a dozen countries have aggressive, government-backed programs to promote the pill. In the U.S., there are problems related to Truvada's high cost, lingering skepticism among some doctors, and low usage rates among black gays and bisexuals who have the highest rates of HIV infection.
"Truvada works," said James Krellenstein, a New York-based activist. "We have to start thinking of it not as a luxury but as an essential public health component of this nation's response to HIV."
A few large U.S. cities are promoting Truvada, often with sexually charged ads. In New York, "Bare It All" was among the slogans urging gay men to consult their doctors. The Los Angeles LGBT Center - using what it called "raw, real language" - launched a campaign to increase use among young Latino and black gay men and transgender women.
"We've got the tools to not only end the fear of HIV, but to end it as an epidemic," said the center's chief of staff, Darrel Cummings. "Those at risk have to know about the tools, though, and they need honest information about them."
In New York, roughly 30 percent of gay and bisexual men are using Truvada now, up dramatically from a few years ago, according to Dr. Demetre Daskalakis, a deputy commissioner of the city's health department.
However, Daskalakis said use among young black and Hispanic men - who account for a majority of new HIV diagnoses - lags behind. To address that, the city is making Truvada readily available in some clinics in or near heavily black and Hispanic neighborhoods.
"We like to go to the root of the problem," said Daskalakis, who personally posed for the "Bare It All" campaign.
According to the Centers for Disease Control and Prevention, Truvada would be appropriate for about 1.2 million people in the U.S. - including sex workers and roughly 25 percent of gay men. Gilead Scientific, Truvada's California-based manufacturer, says there are only about 145,000 active prescriptions for HIV prevention use.
Under federal guidelines, prime candidates for preventive use of Truvada include some gay and bisexual men with multiple sexual partners, and anyone who does not have HIV but has an ongoing sexual relationship with someone who has the virus.
Abroad, a few government health agencies - including those in France, Norway, Belgium, Kenya, South Africa, Brazil, and some Canadian provinces - have launched major efforts to promote preventive use of Truvada or generic alternatives, providing it for free or a nominal charge. In Britain, health officials in Scotland and England recently took steps to provide the medication directly through government-funded programs, though in England it's in the form of a trial limited to 10,000 people.
Truvada was launched in 2004, initially used in combination with other drugs as the basic treatment for people who have HIV, the virus that causes AIDS. It is primarily spread through sex.
Controversy arose in 2012 when the Food and Drug Administration approved Truvada to reduce the risk of getting HIV in the first place, for what's called pre-exposure prophylaxis, or PrEP. It blocks the virus from making copies and taking hold. Critics warned that many gay men wouldn't heed Truvada's once-a-day schedule and complained of its high cost - roughly $1,500 a month.
Gilead offers a payment assistance plan to people without insurance that covers the full cost. Some cities and a few states - including Illinois, Massachusetts, and Washington - also help cover costs. Activists have pressed Gilead to make its copay program more generous in light of its profits from Truvada.
"There's no reason it has to cost so much," said Krellenstein.
--------------------------------
When will the HIV prevention pill go generic? Teva won't say, pointing to a confidential settlement
By Rebecca Robbins @rebeccadrobbins
June 12, 2017
Amid widespread confusion over when the HIV prevention pill will go generic, a spokeswoman for Teva on Monday told STAT that the company has reached a confidential settlement controlling when it can enter the marketplace.
That spokeswoman, Elizabeth DeLuca, declined to say when Teva will launch its generic version of the drug currently sold by Gilead as Truvada, or how much it will charge.
The Food and Drug Administration last week approved Teva's bid to sell the first generic pill for the HIV prevention method known as pre-exposure prophylaxis, or PrEP. That news initially brought excitement and hope in the HIV/AIDS community that the new option would usher in lower prices.
But the celebration among activists and researchers quickly gave way to uncertainty and confusion about when the new generic drug will become available. That question matters because the medication isn't likely to see its price go down without generic competition.
Gilead spokesman Ryan McKeel said the generic version of the drug "will not be immediately available." McKeel said that Gilead's patent for one component of the drug doesn't expire until 2021, and its patent for another part retains exclusivity for pediatric studies until 2018.
Gilead has been fighting with Teva and other would-be generic manufacturers for years over the patents covering Truvada. Gilead and Teva reached a settlement over the patents covering one part of the drug in 2013 and 2014. (It's not clear whether the settlement DeLuca mentioned is different from either of these.)
It's also not clear whether Teva is currently challenging any of Gilead's other patents covering components of Truvada.
In general, when a drug is still under patent, a would-be generic manufacturer must have a challenge underway to the original developer's patent in order to seek FDA approval to sell it too. If the patent-holding company sues the generic applicant but doesn't win within 2 1/2 years, the FDA can go ahead and approve the application. With things still in legal limbo, the generic applicant can decide for itself whether to market the drug before a final decision is reached, according to FDA spokeswoman Lauren Smith Dyer.
It appears that Teva will be able to bypass that route by way of the settlement agreement. Either way, it seems unlikely that hopes for lower-priced generic Truvada will be realized soon.
"We are pleased with the FDA's decision and look forward to supporting these patients with generic medicines when we are otherwise able to," DeLuca said.
---------------------
Gilead's HIV prevention pill will be going generic. How far will Teva drop the price?
By Rebecca Robbins @rebeccadrobbins
June 9, 2017
It's been lauded as an essential public health tool and maligned as "a party drug." And now it's on its way to going generic.
The Food and Drug Administration on Thursday approved Teva's bid to market the first generic form of the daily HIV prevention pill sold as Truvada by Gilead. When it becomes available, it will be closely watched for whether it can meaningfully drive down prices and widen access - which may fuel the debate over the role the medication should play in combating HIV.
Teva got approval to market the drug both to people who are at high risk of HIV and those who already have it, but it wasn't immediately clear when the generic product will become available. Gilead spokesman Ryan McKeel said his company still has patent protection for one component of the drug until 2021. Teva spokeswoman Elizabeth DeLuca declined to comment on when the drug will launch.
She also declined to comment on how much Teva plans to charge.
That's the key question for Dr. Ken Mayer, medical research director at the Boston community clinic Fenway Health, which specializes in LGBT health. He was involved in some of the original studies that got the drug approved for the HIV prevention method known as pre-exposure prophylaxis, or PrEP.
"Given the differential between what PrEP costs when you're providing it to someone in Africa and what it costs if you're paying out of pocket in the U.S., what will be the price point that Teva will be willing to come down to?" Mayer asked.
He added: "If they're underselling Gilead, but not by a lot, it will become a distinction without a difference."
Since Gilead first got approval to sell Truvada as PrEP in 2012, approximately 100,000 Americans are believed to have been prescribed the pill for HIV prevention.
Uptake has gradually increased, but it's still far short of the at-risk Americans - a total of 1.2 million people including sexually active gay men and drug users - the Centers for Disease Control and Prevention recommends should be counseled about possibly taking the drug.
Related Story:
Women's bacteria thwarted attempt at anti-HIV vaginal gel
So why aren't more people taking it? The general practitioners most likely to prescribe the drug to healthy people have little familiarity with it. The at-risk people who make the best candidates for the drug may struggle to access it or keep with the monitoring necessary to take the drug. And cost can be a burden.
Truvada is distributed to high-risk populations overseas by governments and aid organizations at a few hundred dollars a year, but it carries a list price of $18,000 a year in the U.S., according to recent reports. And in America, out-of-pocket costs for people with insurance can be high. Although Gilead does provide assistance programs, "it's complicated and challenging, and a lot of those most at risk - especially young gay and bisexual men - find the barriers too much to overcome," Parsons said.
HIV infection has been on the rise in poor black and Latino communities, places where the challenges around access and insurance coverage are pronounced. "The question is whether cheaper PrEP can move the needle more in those jurisdictions," Mayer said.
The approval for a generic competitor comes at a time when Gilead is looking to more aggressively promote Truvada.
|
|
| |
| |
|
|
|