| |
Association of Immune-Related Adverse Events With Nivolumab Efficacy in Non-Small-Cell Lung Cancer
|
| |
| |
Download the PDF here
Main Outcomes and Measures Kaplan-Meier curves of progression-free survival (PFS) according to the development of irAEs in 6-week landmark analysis were evaluated with the log-rank test as a preplanned primary objective. Overall survival (OS) was similarly evaluated. Multivariable analysis of both PFS and OS was performed with Cox proportional hazard regression models.
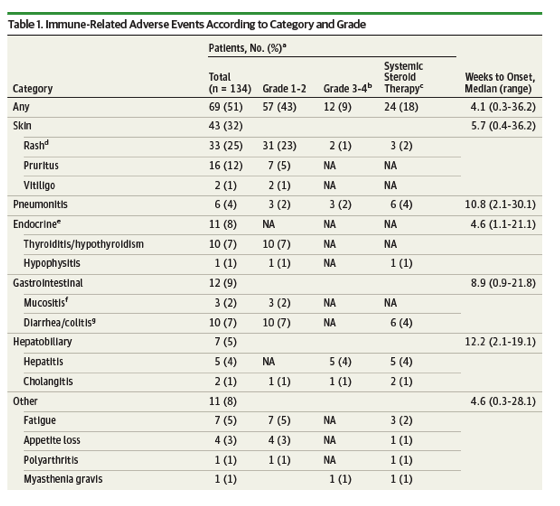
Results In a cohort of 134 patients (median [range] age, 68 [33-85] years; 90 men [67%], 44 women [33%]), irAEs were observed in 69 of the 134 study patients (51%), including 12 patients (9%) with such events of grade 3 or 4, and 24 patients (18%) requiring systemic corticosteroid therapy. In 6-week landmark analysis, median PFS was 9.2 months (95% CI, 4.4 to not reached [NR]) and 4.8 months (95% CI, 3.0 to 7.5) (P = .04) whereas median OS was NR (95% CI, 12.3 to NR) and 11.1 months (95% CI, 9.6 to NR) (P = .01) for patients with or without irAEs, respectively. Multivariable analysis also revealed that irAEs were positively associated with survival outcome, with hazard ratios of 0.525 (95% CI, 0.287 to 0.937; P = .03) for PFS and 0.282 (95% CI, 0.101 to 0.667; P = .003) for OS.
----------------------------------------
Toxicities associated with checkpoint inhibitor immunotherapy
https://www.uptodate.com/contents/toxicities-associated-with-checkpoint-inhibitor-immunotherapy
SUMMARY
⋅ Immunologic checkpoint inhibition agents targeting cytotoxic T-lymphocyte-associated antigen 4 (CTLA-4) and programmed cell death receptor 1 (PD-1) receptor are having a dramatic impact on the care of patients with advanced melanoma and are rapidly being explored as therapy for other malignancies. (See "Immunotherapy of advanced melanoma with immune checkpoint inhibition".)
⋅ Treatment is associated with immune-related adverse events (irAEs) that typically are transient but occasionally can be severe or fatal. The most common and important irAEs are dermatologic, diarrhea/colitis, hepatotoxicity, and endocrinopathies, although other sites can also be affected. (See 'Dermatologic and mucosal toxicity' above and 'Diarrhea/colitis' above and 'Hepatotoxicity' above and 'Endocrinopathies' above and 'Less common immune-related adverse events' above.)
⋅ Rapid identification of irAEs and prompt initiation of local or systemic immunosuppression can optimize outcomes. Side effects are more common with the anti-CTLA-4 antibody ipilimumab than with the anti-PD-1 agents (nivolumab, pembrolizumab) or anti-programmed cell death ligand 1 (PD-L1) agents (atezolizumab, durvalumab, avelumab). The combination of nivolumab plus ipilimumab is associated with more toxicity than either agent alone. (See 'General approach to toxicity management' above.)
⋅ In general, treatment of moderate or severe irAEs requires interruption of the checkpoint inhibitor and the use of corticosteroid immunosuppression [36]. Treatment is based upon the severity of the observed toxicity (see 'General approach to toxicity management' above):
⋅ For patients with grade 2 (moderate) immune-mediated toxicities, treatment with the checkpoint inhibitor should be withheld and should not be resumed until symptoms or toxicity is grade 1 or less. Corticosteroids (prednisone 0.5 mg/kg/day or equivalent) should be started if symptoms do not resolve within a week.
⋅ For patients experiencing grade 3 or 4 (severe or life-threatening) immune-mediated toxicities, treatment with the checkpoint inhibitor should be permanently discontinued. High doses of corticosteroids (prednisone 1 to 2 mg/kg/day or equivalent) should be given. When symptoms subside to grade 1 or less, steroids can be gradually tapered over at least one month.
⋅ If corticosteroids are not effective in treating immunotherapy-related diarrhea after approximately three days, infliximab (5 mg/kg) may be considered. Infliximab should not be given to patients with immune-mediated hepatitis.
⋅ Frequent and consistent communication between patients, caregivers, and the clinical team is vital to successful irAE management.
|
|
| |
| |
|
|
|