| |
Cancers in HIV in Elderly - CD4 / Viral Load, Smoking, Lifestyle
|
| |
| |
Download the PDF here
SEE NEW STUDY FULL TEXT BELOW -"Cancer risk in older people living (>50) with human immunodeficiency virus infection in the United States"
" We studied 183,542 PLWH aged ≥50 years old who contributed 928,194 person-years of follow-up (Table 1)... ..PLWH with a prior AIDS diagnosis had increased KS, NHL (particularly, DLBCL [aIRR=1.97] and CNS lymphomas [aIRR=5.17]), anal, lung, and oral cavity/pharyngeal cancer, and Hodgkin lymphoma rates compared to those who were not previously diagnosed with AIDS (aIRRs ranging from 1.37 to 2.66, Table 3). Prostate cancer rates were lower among those with a prior AIDS diagnosis (aIRR=0.84), while cervical, liver, breast, and colon cancer rates were not significantly different with prior AIDS. Results - We identified 10,371 cancers among 183,542 older PLWH. Risk was significantly increased for KS (SIR=103.34), NHL (SIR=3.05), Hodgkin lymphoma (SIR=7.61), and cervical (SIR=2.02), anal (SIR=14.00), lung (SIR=1.71), liver (SIR=2.91), and oral cavity/pharyngeal (SIR=1.66) cancers, and reduced for breast (SIR=0.61), prostate (SIR=0.47), and colon (SIR=0.63) cancers. SIRs declined with age for all cancers; however, EARs increased with age for anal, lung, liver, and oral cavity/pharyngeal cancers. Cancer risk was highest for most cancers within 5 years after HIV diagnosis; risk decreased with increasing time since HIV diagnosis for KS, NHL, lung cancer, and Hodgkin lymphoma.
Conclusions - Cancer risk is elevated among older PLWH. Although SIRs decrease with age, EARs are higher for some cancers, reflecting a greater absolute excess in cancer incidence among older PLWH. High risk in the first 5 years after HIV diagnosis for some cancers highlights the need for early HIV diagnosis and rapid treatment initiation."
----------------------
Viral Suppression & CD4 in Cancers & Smoking
Effect of immunodeficiency, HIV viral load, and antiretroviral therapy on the risk of individual malignancies (FHDH-ANRS CO4): a prospective cohort study - CD4 & viral load matter: restore or maintain the CD4 count above 500; early treatment initiation - (06/26/13)... ..."The risk of both Kaposi's sarcoma and non-Hodgkin lymphoma increased as the CD4 cell count fell and viral replication rose... ..."At least two-thirds of patients diagnosed with one of the three non-AIDS-defining cancers were receiving cART, with a high frequency of virological efficacy, although the median CD4 count was less than 300 cells per μ L. Patients with anal cancer had a long history of HIV infection, a low nadir CD4 cell count, and a long cumulative period of immunodeficiency or high viral replication, or both; nearly all these patients were on antiretroviral therapy (table 2)... ..The risk of the three non-AIDS-defining cancers increased with decreasing CD4 cell counts (table 4). The risk of Hodgkin's lymphoma increased for CD4 count less than 350 cells per μ L and peaked at 50-99 cells per μ L. The risk of lung cancer was doubled by CD4 counts in the range 350-499 cells per μ L, and continued to increase thereafter as the CD4 cell count fell. The risk of liver cancer was raised at CD4 count less than 500 cells per μ L, and then reached a plateau at less than 200 cells per μ L (table 4). The risk of anal cancer increased with the cumulative duration of CD4 counts less than 200 cells per μ L and with the cumulative duration of viral load more than 5 log10 copies per mL (table 5). A higher CD4 cell count was associated with lower risk of cervical cancer, whereas patients receiving cART were half as likely to develop this cancer""
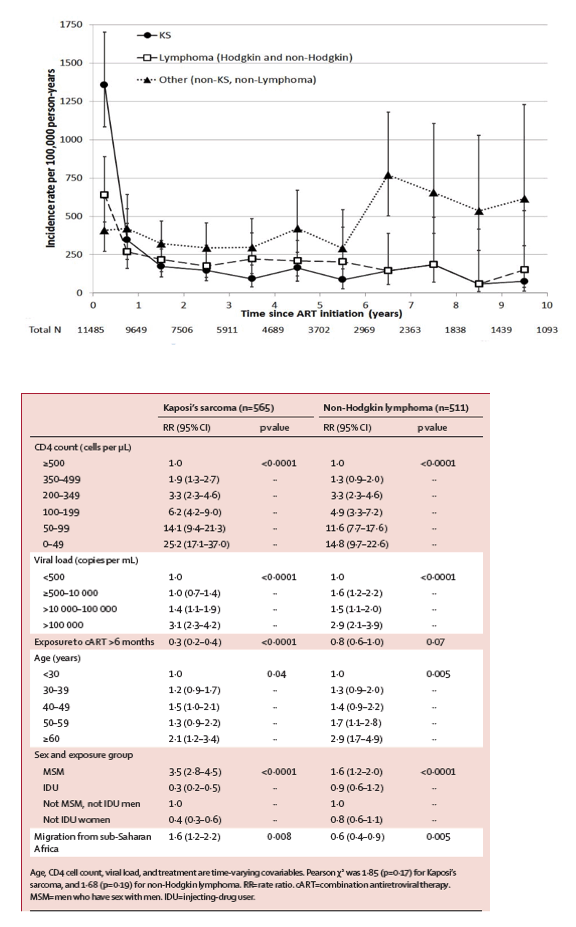
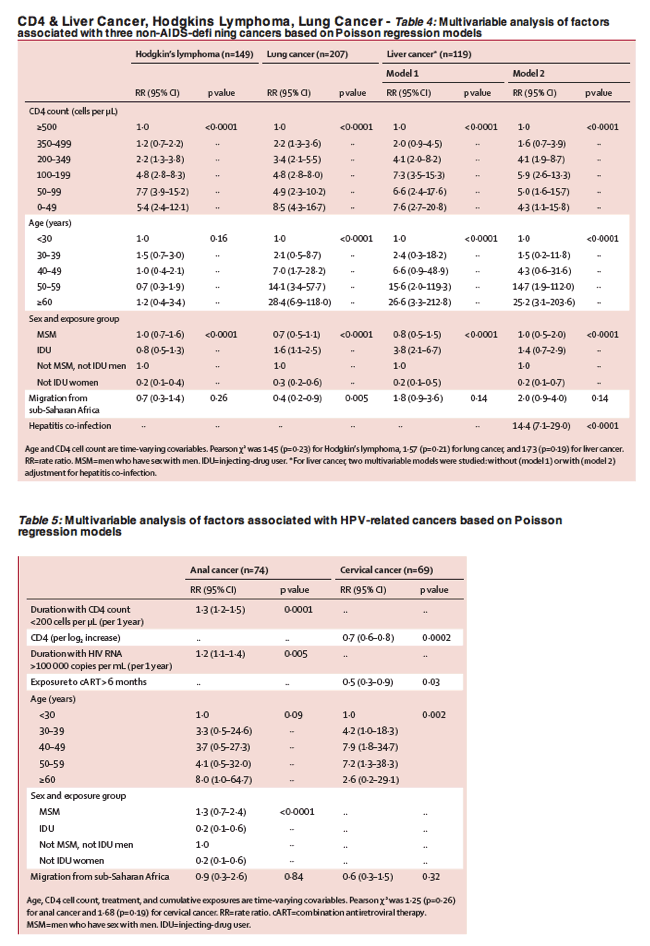
Older age and severe immunodeficiency were associated with an increased risk for all classes of cancer in our study... .."... ... .Regarding the question of 'when to begin HAART', risk for non-AIDS cancers are 2-fold higher (100% higher) when CD4s are below 700 and 3.7 fold higher when CD4 is 200-349 in the pre-HAART era in this study and also 2-fold higher for... ...At the time of first cancer diagnosis, patients with non-virus-related NADCs were older than those with ADCs and with virus-related NADCs (P < 0.001) and they had higher CD4 cell counts (P < 0.001) and higher CD4 nadirs (P < 0.05) (Table 1). Patients with ADCs had the highest HIV viral loads (P < 0.05), and cART was less frequently received in this group (P < 0.001) (Table 1)...... ......Older age and severe immunodeficiency were associated with an increased risk for all classes of cancer in our study. Ageing of HIV-infected patients as a result of the increased life expectancy provided by effective cART has increased the risk of many diseases, including malignancies. The accelerated ageing process that occurs in the immune system, also known as "immunosenescence", during HIV infection could account for the higher risk of cancer in people living with HIV infection. The association of virus-related NADCs with low CD4 cell counts suggests that a defect in immunosurveillance on infection with oncogenic agents is permissive for malignant transformation."... .http://www.natap.org/2013/HIV/080913_03.htm
"http://www.natap.org/2010/HIV/123010_01.htm.......Adjusted hazard of death was lower among those with higher CD4 cell counts at cancer diagnosis, who achieved HIV RNA suppression (<=400 copies/mL) on cART, received any cancer treatment, and had AIDS-defining cancer or infection-related NADCs compared to infection-unrelated NADCsEvery year spent with a CD4 count under 200 independently raised the risk of a non-AIDS cancer diagnosis in the Dutch ATHENA cohort". "This analysis determined that every year with a CD4 count under 200 independently raised the non-AIDS cancer risk about 10% (P = 0.03). There was also a strong trend toward non-AIDS cancer diagnosis for every additional year with a CD4 count between 200 and 350 (P = 0.09). Every year with a CD4 count under 200 independently raised the risk of non-AIDS infection-related cancers about 20% (P = 0.01) but had no significant impact on other non-AIDS cancers. The trend linking time spent with 200 to 350 CD4s and infection-related cancers fell short of statistical significance (P = 0.13).
Every additional 10 years of age (P < 0.0001) and HBV coinfection (P = 0.02) nearly doubled the risk of non-AIDS cancer, and a previous AIDS diagnosis upped the risk about 40% (P = 0.03). Time spent with a viral load above 400 copies did not independently predict cancer."
"This analysis determined that every year with a CD4 count under 200 independently raised the non-AIDS cancer risk about 10% (P = 0.03). There was also a strong trend toward non-AIDS cancer diagnosis for every additional year with a CD4 count between 200 and 350 (P = 0.09). Every year with a CD4 count under 200 independently raised the risk of non-AIDS infection-related cancers about 20% (P = 0.01) but had no significant impact on other non-AIDS cancers. The trend linking time spent with 200 to 350 CD4s and infection-related cancers fell short of statistical significance (P = 0.13).
Every additional 10 years of age (P < 0.0001) and HBV coinfection (P = 0.02) nearly doubled the risk of non-AIDS cancer, and a previous AIDS diagnosis upped the risk about 40% (P = 0.03). Time spent with a viral load above 400 copies did not independently predict cancer."... .http://www.natap.org/2016/IDSA/IDSA_44.htm
CROI: Avoiding smoking could prevent 37% of non-AIDS cancers in adults with HIV....."Smoking Outweighs HIV-related Risk Factors for Non-AIDS-defining Cancers".....'CD4 >200 & viral load matter too in cancer prevention'....modifiable risk factors to prevent cancer - (03/09/15)
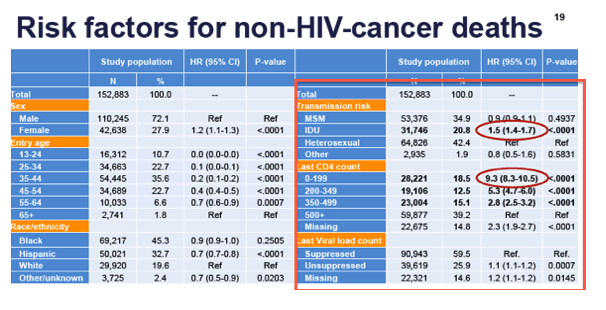
Cancer mortality among persons with Human Immunodeficiency Virus Infection, New York City, 2001-2015 - Lung (most) & Liver Cancer, Breast Cancer, Colorectal Cancers, Non-Hodgkins Lymphoma; RISK FACTORS: low CD4, unsupressed viral load; IDU; Hispanic
http://www.natap.org/2017/IDWeek/IDWeek_47.htm
CD4 & Viral Load... ... ... ... ..
-------------------------------------------------
"In the United States during 2006-2010, HIV-infected people continued to have elevated rates for anal cancer (32-fold elevation compared with the general population), lung cancer (two-fold), liver cancer (three-fold) and Hodgkin lymphoma (10-fold) [29]. In the HAART era, the probability of HIV-infected people in North America developing these cancers by age 65 was 1.3% for anal cancer, 2.2% for lung cancer, 0.8% for liver cancer and 0.9% for Hodgkin lymphoma"... ..Despite dramatic decreases in rates of AIDS-defining cancers, rates of Kaposi sarcoma, NHL and cervical cancer remain elevated 800-fold, 10-fold and four-fold, respectively, compared with the general population [29]. A consortium of North American cohorts estimated that in the HAART era, the probability of developing cancer (i.e. cumulative incidence) by age 65 among HIV-infected people was 4% for both Kaposi sarcoma and NHL, though the cumulative incidence declined significantly across 1996-2009 [33 ]... ..In the United States during 2006-2010, HIV-infected people continued to have elevated rates for anal cancer (32-fold elevation compared with the general population), lung cancer (two-fold), liver cancer (three-fold) and Hodgkin lymphoma (10-fold) [29]. In the HAART era, the probability of HIV-infected people in North America developing these cancers by age 65 was 1.3% for anal cancer, 2.2% for lung cancer, 0.8% for liver cancer and 0.9% for Hodgkin lymphoma [33 ]... .http://www.natap.org/2017/HIV/012717_02.htm
-----------------------------------------
Cancer risk among the HIV-infected elderly (>65) in the United States [AIDS June 19 2016]... ..http://www.natap.org/2016/HIV/060716_02.htm
HIV-infected people and elderly people have higher cancer risk, but the combined effects of aging and HIV are not well described. We aimed to evaluate the magnitude of cancer risk in the HIV-infected elderly population. We conducted a case-cohort study including a 5% sample of U.S. Medicare enrollees and all cancer cases aged at least 65 in linked cancer registries.
Total cancer burden was high among the HIV-infected U.S. elderly, with one out of 10 people getting cancer over 5 years. This reflected an elevated risk for many HIV-associated cancers and a high frequency of cancers that are common among older adults but unassociated with HIV. The resulting cancer distribution reflects both HIV and aging effects. Overall, cancer risk was 50% higher in HIV-infected people than in HIV-uninfected people
Over 5 years, 10.1% of the HIV-infected elderly developed cancer, the most common diagnoses comprising lung (5-year cumulative incidence=2.2%), prostate (2.7%, among men), and colorectal cancer (0.9%), and non-Hodgkin lymphoma (0.8%).
There were 653 cancer cases among HIV-infected people, including 55 AIDS-defining and 598 non-AIDS-defining cancers. Among HIV-infected individuals, 10.1% were diagnosed with cancer over the course of 5 years of follow-up [95% confidence interval (CI) = 8.7-11.5%, Fig. 1]. The most common cancer was lung cancer (5-year cumulative incidence = 2.2%), followed by prostate cancer (1.9%), colorectal cancer (0.9%), NHL (0.8%), and anal cancer (0.6%) (Fig. 1).
Among HIV-infected men, 11.5% (95%CI = 9.6-13.3%) were diagnosed with cancer over 5 years, whereas 6.7% (95%CI = 4.7-8.6%) of HIV-infected women were diagnosed with cancer over 5 years (Fig. 1). The most frequent cancers among men were prostate cancer (5-year cumulative incidence = 2.7%), lung cancer (2.4%), NHL (0.9%), colorectal cancer (0.9%), and anal cancer (0.8%). Among women, the most frequent cancers were lung cancer (5-year cumulative incidence = 1.6%), colorectal cancer (1.0%), breast cancer (1.0%), NHL (0.4%), and pancreatic cancer (0.3%).
Cancer incidence was approximately 50% higher in HIV-infected compared with HIV-uninfected individuals (aHR = 1.52, 95%CI = 1.32-1.75; Table 1). HIV was most strongly associated with Kaposi sarcoma (aHR = 94.4, 95%CI = 54.6-163). HIV was also associated with elevated incidence of anal cancer (aHR = 34.2), Hodgkin lymphoma (aHR = 6.30), liver cancer (aHR = 3.35), NHL (aHR = 2.55), oral cavity/pharyngeal cancer (aHR = 1.79), and lung cancer (aHR = 1.61; Table 1). HIV was inversely associated with prostate cancer (aHR = 0.78, 95%CI = 0.63-0.98).
For NHL, associations were strongest for the AIDS-defining subtypes, which comprised 65% of NHLs in HIV-infected people. Central nervous system lymphoma was most strongly associated with HIV (aHR = 15.3, 95%CI = 6.81-34.6). Incidence was also elevated for diffuse large B-cell lymphoma (aHR = 5.61) and Burkitt lymphoma (aHR = 18.8; Table 1). Other specified lymphomas were not associated with HIV (aHR = 0.74, 95%CI = 0.40-1.35), though they made up 20% of NHL cases among HIV-infected people.
Prostate cancer incidence of both localized/regional and distant stage cases appeared decreased among HIV-infected people, though the association was only significant for localized/regional prostate cancer (aHR = 0.74, 95%CI = 0.59-0.94; Table 1). HIV was not associated with breast cancer incidence at any stage. Incidence of localized and distant colorectal cancer was the same in individuals with and without HIV, whereas incidence of regional stage colorectal cancer was elevated among HIV-infected people (aHR = 1.70, 95%CI = 1.12-2.58).
------------------------------
this below is a detailed study with subtle nuances on which cancer are increased in risk and which are down in risks for older HIV+ or for HIV+ in general vs the general population. So its important to read through the text I inserted just below & the tables just following the text to understand the risks. Rates in general for cancers in HIV+ are not high, they are low, but they are higher compared to the general population.
Cancer risk in older people (>50) living with human immunodeficiency virus infection in the United States
CID Jan 8 2018
Cancer risk is increased in people living with HIV (PLWH). Improved survival has led to an aging of PLWH. We evaluated the cancer risk in older PLWH (age ≥50 years). We included data from the HIV/AIDS Cancer Match Study (1996-2012) and evaluated risk of Kaposi sarcoma (KS), non-Hodgkin lymphoma (NHL), Hodgkin lymphoma, and cervical, anal, lung, liver, oral cavity/pharyngeal, breast, prostate, and colon cancers in older PLWH compared to the general population by calculating the standardized incidence ratios (SIRs) and excess absolute risks (EARs). Cancer risk by time since HIV diagnosis was estimated using Poisson regression
In conclusion, rates of several cancer types are elevated in older PLWH compared to the general population, albeit in the absence of information on important cancer risk factors. However, the relative risk of cancers is lower than for younger PLWH, suggesting that the combined effect of aging and HIV does not further amplify the relative risk of cancers. Despite lower relative risks, the absolute risk difference is higher for some NADCs among older PLWH leading to a greater incidence of excess cancers in this group. Thus, there is a continued need for cancer prevention and early detection among older PLWH. Cancer risk was also highest within the first 5 years after HIV diagnosis for most cancers, underscoring the importance of early HIV diagnosis, rapid initiation of HAART after HIV diagnosis, and interventions to reduce traditional risk factors in older PLWH.
Results
We identified 10,371 cancers among 183,542 older PLWH. Risk was significantly increased for KS (SIR=103.34), NHL (SIR=3.05), Hodgkin lymphoma (SIR=7.61), and cervical (SIR=2.02), anal (SIR=14.00), lung (SIR=1.71), liver (SIR=2.91), and oral cavity/pharyngeal (SIR=1.66) cancers, and reduced for breast (SIR=0.61), prostate (SIR=0.47), and colon (SIR=0.63) cancers. SIRs declined with age for all cancers; however, EARs increased with age for anal, lung, liver, and oral cavity/pharyngeal cancers. Cancer risk was highest for most cancers within 5 years after HIV diagnosis; risk decreased with increasing time since HIV diagnosis for KS, NHL, lung cancer, and Hodgkin lymphoma.
Conclusions
Cancer risk is elevated among older PLWH. Although SIRs decrease with age, EARs are higher for some cancers, reflecting a greater absolute excess in cancer incidence among older PLWH. High risk in the first 5 years after HIV diagnosis for some cancers highlights the need for early HIV diagnosis and rapid treatment initiation.
Given the aging of PLWH and the increase in the burden of NADCs, it is essential to target available cancer prevention and screening strategies towards this population. Cancer prevention among PLWH is largely based on restoring immunity with HAART and reducing traditional cancer risk factors [36]. Timely linkage of care with HAART initiation and uninterrupted treatment for people who have been newly diagnosed with HIV infection is essential to restore immunity. This also gives an opportunity for healthcare providers to diagnose and treat coinfections, and to initiate counseling on modification of lifestyle behaviors such as smoking. General population screening guidelines for breast, prostate, and colon cancers should be followed. Cervical cancer screening is recommended at more frequent intervals than for HIV-uninfected women [37, 38], and the benefits of anal cancer screening are still being evaluated [37, 38]. Finally, screening for lung cancers with low-dose computerized tomography may be beneficial, but more data are needed among PLWH [37, 38].
Although the risk of anal, lung, liver, and oral cavity/pharyngeal cancers in PLWH relative to the general population decreased with age, there was an increase in the excess cancer rates in absolute terms, as reflected in the EARs. Due to increasing cancer incidence in the general population with age, even a modest decrease in SIRs with advancing age could lead to an increase in EARs and thus number of cancer cases. Thus, as PLWH continue to age, we may continue to observe an increase in the absolute number of these NADCs.
Cancer risk is increased in people living with HIV (PLWH). Improved survival has led to an aging of PLWH. We evaluated the cancer risk in older PLWH (age ≥50 years)... ... ..With the success of HAART, HIV infection has transformed from a terminal illness to a more manageable chronic disease. Consequently, more PLWH are reaching older ages. In our analyses that included more than 180,000 older PLWH, we observed that cancer risk was elevated in older PLWH compared to the general population, though the relative risk for most cancers declined with age. However, EARs, which measure absolute risk and thus reflect the number of excess cancers occurring among PLWH, increased with age for some NADCs. We also observed that for many cancers, cancer risk was highest within the first five years after HIV diagnosis.
In the past 20 years, there has been a steady shift in the demographic profile of the HIV/AIDS epidemic in the U.S. towards the older age groups. In 2002, the CDC estimated that approximately 8.5% of the PLWH were over 50 years of age, and that number had increased to 44% in 2014 [12]. With aging, there is a complex interaction between various biological processes which may modulate cancer risk among PLWH: (i) factors related to aging, including age-related immune senescence; (ii) factors associated with HIV infection and its related immune dysfunction, such as HIV viral load, CD4+ T-cell count, and effectiveness of HAART; and (iii) various host factors that may affect cancer risk, such as viral coinfections and smoking. Furthermore, the immunosuppressive state in PLWH changes over time. The natural course of HIV infection is characterized by progressive decline in immune function from primary HIV infection to symptomatic disease with onset of AIDS-defining conditions [23]. HAART alters the natural history of HIV infection with gradual improvement of immunosuppressive state and restoration of CD4+ T-cell count over time. In addition, the duration of immunosuppressed state of a person infected with HIV also increases with time.
RESULTS & TABLES EXplained... ... .
We studied 183,542 PLWH aged ≥50 years old who contributed 928,194 person-years of follow-up (Table 1). The person-time distribution was: 75.1% in 50-59-year-olds, 20.4% in 60-69-year-olds, and 4.5% in 70+-year-olds. A large fraction of person-time was contributed by non-Hispanic blacks (47.6%), men (74.3%), and those with a prior AIDS diagnosis (71.9%). A large proportion of person-time for the time since HIV diagnosis was unknown (22.2%). Among PLWH with a known date of HIV diagnosis, more than 60% were infected with HIV for at least 5 years and approximately 20% were living with HIV infection for more than 15 years.
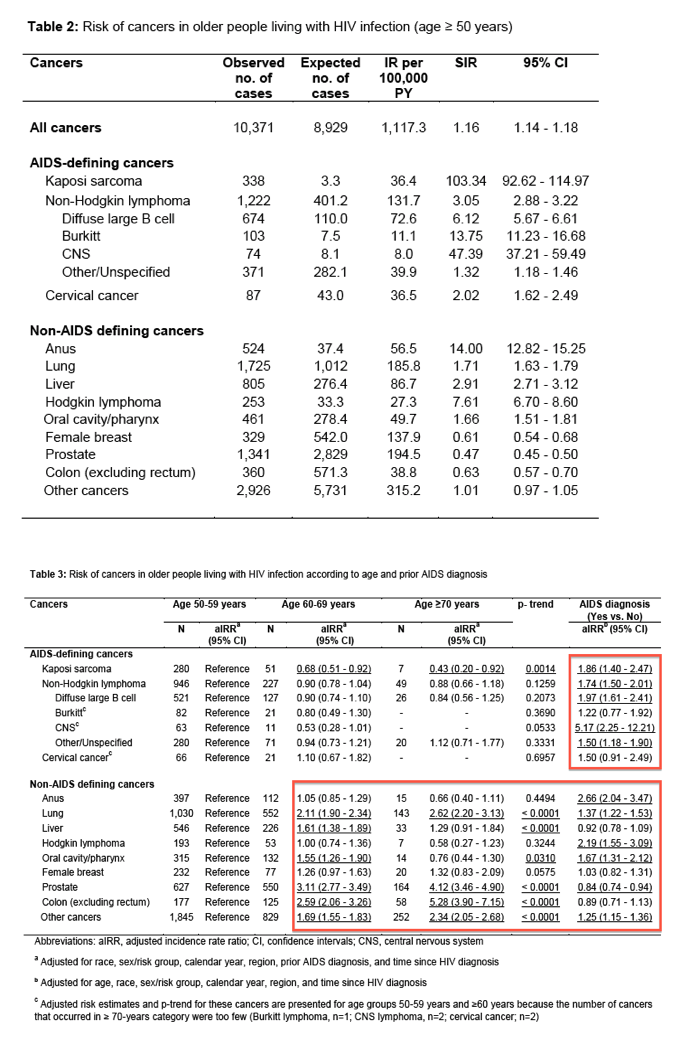
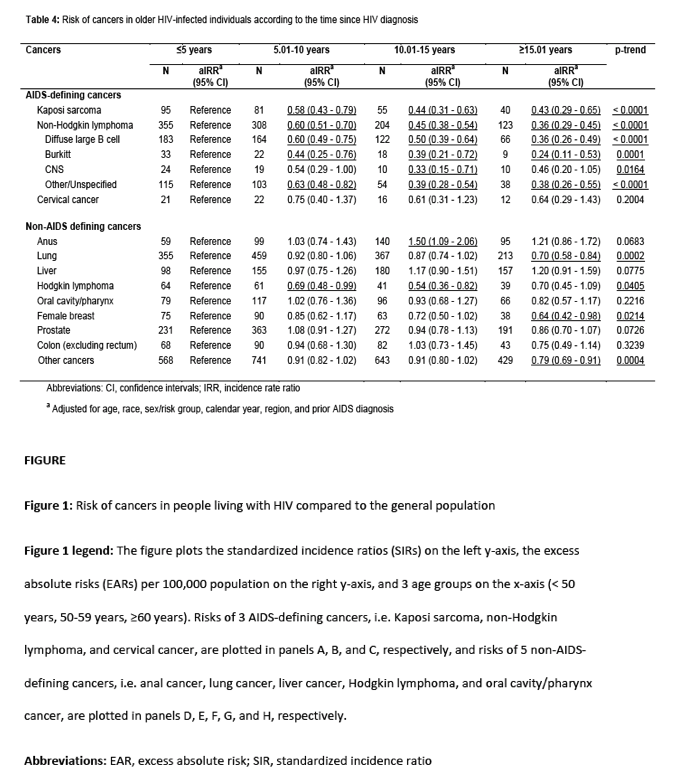
During the follow-up, 10,371 cancers were diagnosed, of which 1,647 (15.9%) were ADCs and 8,724 (84.1%) were NADCs (Table 2). As expected, rates were increased in older PLWH compared to the general population for KS (SIR=103.34), total NHL (SIR=3.05), and cervical cancer (SIR=2.02). Among NHLs, rates were strongly elevated for CNS lymphoma (SIR=47.39) and Burkitt lymphoma (SIR=13.75). Among the NADCs, a very high risk of anal cancer (SIR=14.00) was observed followed by Hodgkin lymphoma (SIR=7.61). Rates were moderately increased for liver (SIR=2.91), lung (SIR=1.71), and oral cavity/pharyngeal cancers (SIR=1.66). Furthermore, breast (SIR=0.61), prostate (SIR=0.47), and colon cancer rates (SIR=0.63) were significantly lower among PLWH than the general population. The SIRs did not differ appreciably after adjusting the person-time for late follow-up, or by restricting analyses to calendar years 2001-2012 (Supplementary Tables 2 and 4).
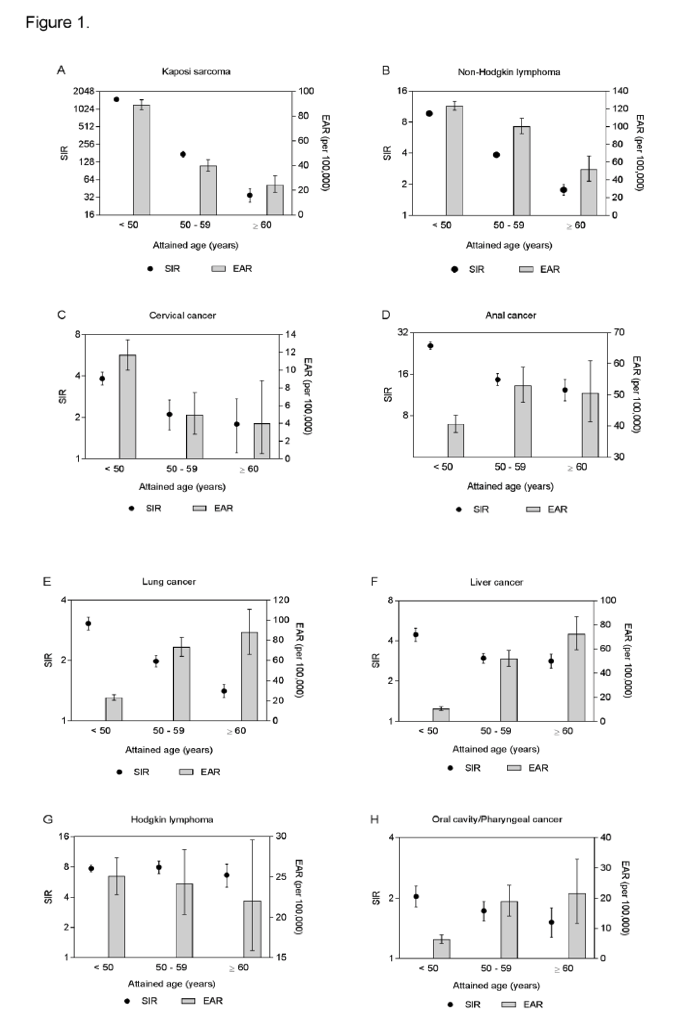
A comparison of SIRs and EARs by age for evaluated cancers is depicted in Figure 1. The SIRs were the highest for the youngest age group (<50 years) for all cancers and decreased with age, except for Hodgkin lymphoma, for which the SIRs were largely constant. The EARs also decreased with age for KS, NHL, cervical cancer, and Hodgkin lymphoma. In contrast, the EARs increased with age for cancers of the anus, lung, liver, and oral cavity/pharynx.
When cancer incidence rates were examined by age group among PLWH (Table 3), we observed a significant decrease in KS risk with increasing age. When compared to 50-59-year-olds, PLWH who were 60-69 (aIRR=0.68) or ≥70 years old (aIRR=0.43) had lower KS rates (p-trend=0.0014). In contrast, rates for lung, prostate, and colon cancers increased with age (p-trend<0.0001) while the trend for breast cancer was borderline significant (p-trend=0.0575). Liver and oral cavity/pharyngeal cancer rates were higher among 60-69-year-olds compared to the 50-59-year age group (p-trend<0.05). No association between age and cancer risk was observed for NHL (total or subtypes), cervical cancer, anal cancer, or Hodgkin lymphoma (p-trend>0.05).
PLWH with a prior AIDS diagnosis had increased KS, NHL (particularly, DLBCL [aIRR=1.97] and CNS lymphomas [aIRR=5.17]), anal, lung, and oral cavity/pharyngeal cancer, and Hodgkin lymphoma rates compared to those who were not previously diagnosed with AIDS (aIRRs ranging from 1.37 to 2.66, Table 3). Prostate cancer rates were lower among those with a prior AIDS diagnosis (aIRR=0.84), while cervical, liver, breast, and colon cancer rates were not significantly different with prior AIDS.
Risk of most cancers was highest within the first 5 years after HIV diagnosis, and gradually decreased over time (Table 4). Significant decreasing trends were observed for KS (p-trend<0.0001), total NHL (p-trend<0.0001), lung cancer (p-trend=0.0002), breast cancer (p-trend=0.02), and Hodgkin lymphoma (p-trend=0.04), while a marginally statistically significant trend was observed for prostate cancer (p-trend=0.07). In contrast, there was an indication that risk increased with increasing time since HIV diagnosis for anal (p-trend=0.07) and liver cancers (p-trend=0.08). Excluding the first year after HIV diagnosis did not affect our results (Supplementary Table 3). Restricting the analyses to calendar years 2001-2012 did not affect the associations between age or time since HIV diagnosis and cancer risk (Supplementary Tables 5 and 6).
The associations between age and cancer risk did not differ by time since HIV diagnosis for all cancers (all p-interaction>0.05). However, there was significant heterogeneity in the association between time since HIV diagnosis and cancer risk by age for NHL (p-interaction=0.0026) and Hodgkin lymphoma (p-interaction=0.0211). The ratios for NHL decreased with time since HIV diagnosis for both age categories (p-trend<0.0001 for ages 50-59 and ≥60 years). For Hodgkin lymphoma, the trend for decreasing rates with time since HIV diagnosis was only observed for ≥60-year-olds (p-trend=0.0063), but not among those who were 50-59 years old (p-trend=0.4065).
------------------------------
New study published
Cancer risk in older people (>50) living with human immunodeficiency virus infection in the United States
Clin Inf Dis Jan 8 2018 - Parag Mahale,1 Eric A. Engels,1 Anna E. Coghill,1 Amy R. Kahn,2 Meredith S. Shiels1
1Division of Cancer Epidemiology & Genetics, National Cancer Institute, Rockville, MD
2New York State Cancer Registry, New York State Department of Health, Albany, NY
Abstract
Background
Cancer risk is increased in people living with HIV (PLWH). Improved survival has led to an aging of PLWH. We evaluated the cancer risk in older PLWH (age ≥50 years).
Methods
We included data from the HIV/AIDS Cancer Match Study (1996-2012) and evaluated risk of Kaposi sarcoma (KS), non-Hodgkin lymphoma (NHL), Hodgkin lymphoma, and cervical, anal, lung, liver, oral cavity/pharyngeal, breast, prostate, and colon cancers in older PLWH compared to the general population by calculating the standardized incidence ratios (SIRs) and excess absolute risks (EARs). Cancer risk by time since HIV diagnosis was estimated using Poisson regression.
Results
We identified 10,371 cancers among 183,542 older PLWH. Risk was significantly increased for KS (SIR=103.34), NHL (SIR=3.05), Hodgkin lymphoma (SIR=7.61), and cervical (SIR=2.02), anal (SIR=14.00), lung (SIR=1.71), liver (SIR=2.91), and oral cavity/pharyngeal (SIR=1.66) cancers, and reduced for breast (SIR=0.61), prostate (SIR=0.47), and colon (SIR=0.63) cancers. SIRs declined with age for all cancers; however, EARs increased with age for anal, lung, liver, and oral cavity/pharyngeal cancers. Cancer risk was highest for most cancers within 5 years after HIV diagnosis; risk decreased with increasing time since HIV diagnosis for KS, NHL, lung cancer, and Hodgkin lymphoma.
Conclusions
Cancer risk is elevated among older PLWH. Although SIRs decrease with age, EARs are higher for some cancers, reflecting a greater absolute excess in cancer incidence among older PLWH. High risk in the first 5 years after HIV diagnosis for some cancers highlights the need for early HIV diagnosis and rapid treatment initiation.
INTRODUCTION
People living with human immunodeficiency virus (HIV) infection (PLWH) have an increased cancer risk due to HIV-induced immunosuppression, co-infections with oncogenic viruses, and high prevalence of behavioral risk factors for cancer such as smoking [1-10]. Besides the AIDS-defining cancers (ADCs), i.e. Kaposi sarcoma (KS), certain non-Hodgkin lymphomas (NHLs), and cervical cancer, the risks of some non-AIDS defining cancers (NADCs), including lung, liver, anal, and oral cavity/pharyngeal cancers, and Hodgkin lymphoma are also increased [2, 5-10].
The introduction of highly active antiretroviral therapy (HAART) has led to a steady increase in the life expectancy of PLWH [11]. Consequently, the number of older PLWH in the US has also increased. In 2014, approximately 44% PLWH in the US were aged ≥50 years old, and 17% of new HIV diagnoses were made among ≥50 year-olds [12, 13]. Because of improved immunity due to HAART, rates of ADCs have declined over the past two decades [7, 14, 15]. However, rates of many NADCs increase with age. It is unclear whether age-related immune senescence in the setting of HIV-related immunosuppression will result in particularly elevated cancer rates among older PLWH.
PLWH aged ≥50 years are a heterogeneous group that includes long-term survivors who have lived with HIV infection for many years, and newly diagnosed individuals; whether cancer risk varies based on duration of HIV infection is uncertain. Prolonged immunosuppression in those with long-term HIV infection may increase cancer risk. Alternatively, people newly diagnosed with HIV infection at an older age may have more severe immunosuppression and a consequent high risk for cancer. People who are diagnosed with HIV at older ages may present late with a lower CD4+ T-cell count, later HAART initiation, and more rapid progression to AIDS than young PLWH [16-19].
As the proportion of older PLWH continues to grow, a comprehensive evaluation of cancer risk in this population is needed. We therefore examined cancer risk in older PLWH compared to the general population, and evaluated the effects of age and time since HIV diagnosis on cancer risk.
SUBJECTS AND METHODS
Study population and outcomes
The U.S. HIV/AIDS Cancer Match (HACM) study links data from population-based HIV and cancer registries using a probabilistic algorithm (https://hivmatch.cancer.gov) [14]. This analysis included data from 9 U.S. states/territories (See Table 1 note). The HACM Study was approved by the institutional review boards at the National Cancer Institute and, as required, participating cancer and HIV registries.
The HIV diagnosis date was identified from HIV registries as the date of the first available positive serological test for HIV infection. The follow-up for each person started at the later of the start of cancer registry coverage, 4 months after the HIV report date (or AIDS diagnosis date if earlier), or age 50 years, and ended at the earlier of death or end of cancer registry coverage.
We evaluated risk of ADCs: KS, cervical cancer, and NHL (overall and by subtypes: diffuse large B cell lymphoma [DLBCL], Burkitt lymphoma, primary central nervous system [CNS] lymphoma, and other/unspecified NHLs); and certain NADCs which have increased incidence among PLWH: cancers of the anus, lung, liver, and oral cavity/pharynx, and Hodgkin lymphoma [5]. Female breast, colon, and prostate cancers were also included; though their rates increase with age, these cancers are not elevated among PLWH [5]. The remaining cancers were grouped together in a separate category. Cancers were classified by using the International Classification of Diseases for Oncology (3rd Edition) topography and morphology codes for invasive cancers (See Supplemental Table 1) [20].
Statistical analyses
We compared cancer incidence rates between PLWH and the general population by calculating standardized incidence ratios (SIRs), which measures relative risk, and excess absolute risks (EARs), which measures absolute risk. SIRs were calculated as observed divided by the expected number of cases; expected cases were estimated by applying general population cancer incidence rates to the HIV population by age, sex, race/ethnicity, calendar year, and cancer registry. Since the AIDS epidemic affected the contemporaneous rates of KS and primary CNS lymphoma in the general population, we calculated the expected number of cases for these cancers with pre-AIDS epidemic (1973-1979) general population cancer incidence rates obtained from the Surveillance, Epidemiology, and End Results (SEER) Program, stratified by race, sex, and age [21]. EARs for each cancer were estimated as the difference between the observed and expected cancer rates for age groups 50-59 and ≥60 years. For comparison, we also evaluated the SIRs and EARs among PLWH <50 years of age.
Among PLWH, we estimated cancer risk according to age (50-59, 60-69, ≥70 years), AIDS diagnosis (yes/no), and time since HIV diagnosis (≤5, 5.01-10, 10.01-15, and ≥15.01 years) by fitting Poisson regression models for each cancer type. Incidence rate ratios were adjusted (aIRRs) for race/ethnicity, sex/risk group, calendar year, region, and prior AIDS diagnosis and time since HIV diagnosis (in models for age) or age (in models for time since HIV diagnosis). Age, calendar year, prior AIDS diagnosis, and time since HIV diagnosis variables were updated over time.
We conducted some sensitivity analyses. Since outmigration of people from registry areas may result in over-estimating the person-time at risk, we decreased the person-time by 10% for PLWH after 10 years of follow-up and recalculated the SIRs [22]. It is possible that more cancer cases may be diagnosed shortly after HIV diagnosis because there may be a period of increased medical care around the time of HIV diagnosis. Hence, we excluded the first year after HIV diagnosis date and re-estimated cancer risk according to time since HIV diagnosis. As risk of ADCs and NADCs have changed since the introduction of HAART, we restricted the follow-up time to calendar years 2001-2012 and recalculated the SIRs and aIRRs. Finally, we evaluated whether associations between age and cancer risk differed by time since HIV diagnosis, and associations between time since HIV diagnosis and cancer risk differed by age.
DISCUSSION
With the success of HAART, HIV infection has transformed from a terminal illness to a more manageable chronic disease. Consequently, more PLWH are reaching older ages. In our analyses that included more than 180,000 older PLWH, we observed that cancer risk was elevated in older PLWH compared to the general population, though the relative risk for most cancers declined with age. However, EARs, which measure absolute risk and thus reflect the number of excess cancers occurring among PLWH, increased with age for some NADCs. We also observed that for many cancers, cancer risk was highest within the first five years after HIV diagnosis.
In the past 20 years, there has been a steady shift in the demographic profile of the HIV/AIDS epidemic in the U.S. towards the older age groups. In 2002, the CDC estimated that approximately 8.5% of the PLWH were over 50 years of age, and that number had increased to 44% in 2014 [12]. With aging, there is a complex interaction between various biological processes which may modulate cancer risk among PLWH: (i) factors related to aging, including age-related immune senescence; (ii) factors associated with HIV infection and its related immune dysfunction, such as HIV viral load, CD4+ T-cell count, and effectiveness of HAART; and (iii) various host factors that may affect cancer risk, such as viral coinfections and smoking. Furthermore, the immunosuppressive state in PLWH changes over time. The natural course of HIV infection is characterized by progressive decline in immune function from primary HIV infection to symptomatic disease with onset of AIDS-defining conditions [23]. HAART alters the natural history of HIV infection with gradual improvement of immunosuppressive state and restoration of CD4+ T-cell count over time. In addition, the duration of immunosuppressed state of a person infected with HIV also increases with time.
Few previous studies could evaluate cancer risk in older PLWH as this population has historically been too small to target [24, 25]. In our study, we observed that the risk of ADCs and certain NADCs were elevated in older PLWH compared to the general population, as has been previously reported in mostly young PLWH [3, 7, 26]. Because cancer risk increases with age, and PLWH are at a high risk for cancer, the aging of PLWH raises the question of whether the combined effect of age and HIV infection will further increase cancer risk in PLWH. As captured by SIRs, we did not observe an amplified cancer risk among PLWH associated with aging. In fact, the SIRs were highest for the young PLWH and declined with age, except for Hodgkin lymphoma, suggesting that HIV-related immunosuppression may play a larger role in cancer development in younger age groups.
Although the risk of anal, lung, liver, and oral cavity/pharyngeal cancers in PLWH relative to the general population decreased with age, there was an increase in the excess cancer rates in absolute terms, as reflected in the EARs. Due to increasing cancer incidence in the general population with age, even a modest decrease in SIRs with advancing age could lead to an increase in EARs and thus number of cancer cases. Thus, as PLWH continue to age, we may continue to observe an increase in the absolute number of these NADCs.
Risks for KS, NHLs, Hodgkin lymphoma, lung, and breast cancer were highest within the first 5 years after HIV diagnosis. The intensity of immunosuppression may be highest in the initial period, as newly diagnosed PLWH are untreated and may be especially immunosuppressed [27, 28]. Alternatively, it is possible that people who survived for several years with HIV infection may represent a healthy subset of PLWH, while those with newly acquired and diagnosed HIV may have a higher prevalence of cancer risk factors. For example, a diagnosis of HIV infection may lead to changes in lifestyle habits such as reducing cigarette smoking [29]. Increased medical surveillance shortly after HIV diagnosis may also increase the possibility of detecting cancers, but excluding the first year of follow-up after HIV diagnosis did not eliminate the trend that we observed. In contrast, anal and liver cancer rates marginally increased with time since HIV diagnosis, which may represent the role of prolonged immunosuppression in increasing cancer rates due to human papillomavirus or hepatitis virus co-infections.
We observed lower risk of breast, prostate, and colon cancer rates in older PLWH compared to the general population, as has been previously reported [26, 30-33]. As in the general population, prostate and colon cancer rates increased with age, and the trend for breast cancer was borderline significant. As these are common screen-detectable cancers, it has been speculated that reduced rates of these cancers may reflect lower rates of cancer screening among PLWH compared with the general population. However, recent studies suggest that the deficit of breast, colon, and prostate cancers among PLWH cannot be completely explained by lower rates of cancer screening [34, 35]. Biological explanations for these deficits need to be explored further.
Given the aging of PLWH and the increase in the burden of NADCs, it is essential to target available cancer prevention and screening strategies towards this population. Cancer prevention among PLWH is largely based on restoring immunity with HAART and reducing traditional cancer risk factors [36]. Timely linkage of care with HAART initiation and uninterrupted treatment for people who have been newly diagnosed with HIV infection is essential to restore immunity. This also gives an opportunity for healthcare providers to diagnose and treat coinfections, and to initiate counseling on modification of lifestyle behaviors such as smoking. General population screening guidelines for breast, prostate, and colon cancers should be followed. Cervical cancer screening is recommended at more frequent intervals than for HIV-uninfected women [37, 38], and the benefits of anal cancer screening are still being evaluated [37, 38]. Finally, screening for lung cancers with low-dose computerized tomography may be beneficial, but more data are needed among PLWH [37, 38].
A strength of our study was the use of a large population-based cohort of more than 180,000 PLWH aged ≥50 years with systematic cancer ascertainment, and the evaluation of cancer risk by time since HIV diagnosis. However, we were unable to include information on HAART use, CD4+ T-cell count, or HIV viral load in our analyses. Therefore, we cannot definitively interpret trends with respect to time since HIV diagnosis. In addition, our data do not include information about cancer risk factors, such as cigarette smoking and viral co-infection. Since the timing of acquiring HIV infection was not known, HIV duration could be calculated only from the first available positive serological test date, and 22.2% of person-time contributed by PLWH in our study was missing this information. We were unable to adjust for competing risks due to coexisting comorbidities in our analyses. We used general population as our control group because we did not have access to an ideal HIV-uninfected population which may have similar characteristics to PLWH [39]. Finally, this analysis includes only PLWH who have been diagnosed with HIV, and an estimated 13% of PLWH are unaware that they are infected, more than 7% of whom may be over 50 years of age [40].
In conclusion, rates of several cancer types are elevated in older PLWH compared to the general population, albeit in the absence of information on important cancer risk factors. However, the relative risk of cancers is lower than for younger PLWH, suggesting that the combined effect of aging and HIV does not further amplify the relative risk of cancers. Despite lower relative risks, the absolute risk difference is higher for some NADCs among older PLWH leading to a greater incidence of excess cancers in this group. Thus, there is a continued need for cancer prevention and early detection among older PLWH. Cancer risk was also highest within the first 5 years after HIV diagnosis for most cancers, underscoring the importance of early HIV diagnosis, rapid initiation of HAART after HIV diagnosis, and interventions to reduce traditional risk factors in older PLWH.
|
|
| |
| |
|
|
|