| |
HIV: ageing, cognition and neuroimaging at 4-year follow-up -
'Accelerated Brain Aging'
|
| |
| |
Download the PDF here
"In conclusion, we found independent (main) effects of both HIV status and age group on longitudinal change in cognitive performance and change through time in mean diffusivity using DTI, but not an effect on volumetrics. The results suggested accelerated ageing in HIV infection, in that there was a significant HIV status by age group interaction for change in global cognition. Being HIV positive and older was associated with a worsening in overall cognitive performance. However, this interaction could not be explained by the imaging findings, as there were no significant interactions for any of the imaging metrics. Further work is needed to look at other biological factors that could explain change in overall cognitive performance in HIV infection, including metabolic findings and treatment effects."
from Jules: this study failed to recognize recent publications this year finding HIV accelerates brain aging:
HIV prematurely ages the brain - new study & Commentary - (07/19/17) - "Effect of ageing on neurocognitive function by stage of HIV infection: evidence from the Multicenter AIDS Cohort Study"
Progressive Brain Atrophy Despite Persistent Viral Suppression in HIV Over Age 60 - (07/19/17)
HIV serostatus was associated with more rapid average annualized rates of atrophy in the cerebellum (0.42% vs. 0.02%, p=0.016), caudate (0.74% vs. 0.03%, p=0.012), frontal lobe (0.48% vs. 0.01%, p=0.034), total cortical gray matter (0.65% vs. 0.16%, p=0.027), brain stem (0.31% vs. 0.01%, p=0.026), and pallidum (0.73% vs. 0.39%, p=0.046). Our cross-sectional analyses confirm limited volumetric reductions in HIV-infected participants with cognitive impairment compared to healthy controls, including regions previously noted to be affected in the setting of HIV, such as the cerebellum, nucleus accumbens and brainstem.....By virtue of the older age of our study participants compared to other studies, they may be particularly vulnerable to faster changes in brain atrophy as age-associated brain atrophy rates do not appear to have linear slopes in healthy aging.42 Furthermore, our data examined participants in age over 60 years, surpassing age set points thought to represent the 'tipping point' from linear to faster atrophy in presumed healthy aging. For example, a study of 1100 healthy elders noted nonlinear atrophy trajectories dominate in most subcortical regions after age 60 years.3....One could speculate parallels in HAND and Alzheimer's disease among older study participants as it demonstrates similarly affected subcortical structures..
....................................
HIV: ageing, cognition and neuroimaging at 4-year follow-up
HIVMedicine Feb 14 2018 - BI Haynes ,1 M Pitkanen,1,2 R Kulasegaram,3 SJ Casey,1 M Schutte,1 K Towgood,1 B Peters,1,2 GJ Barker1 and
MD Kopelman1,2
1King's College London (Institute of Psychiatry, Psychology and Neuroscience), London, UK, 2South London and Maudsley
NHS Trust based at St Thomas' Hospital, London, UK and 3Guy's and St Thomas' NHS Trust, St Thomas' Hospital,London, UK
Abstract
Objectives
The aim of the study was to investigate the hypothesis of accelerated cognitive ageing in HIV-positive individuals using longitudinal assessment of cognitive performance and quantitative magnetic resonance imaging (MRI).
Methods
We assessed a broad cognitive battery and quantitative MRI metrics [voxel-based morphometry (VBM) and diffusion tensor imaging (DTI)] in asymptomatic HIV-positive men who have sex with men (15 aged 20-40 years and 15 aged ≥ 50 years), and HIV-seronegative matched controls (nine aged 20-40 years and 16 aged ≥ 50 years).
-------------------------
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2041910/
Diffusion tensor imaging (DTI) is a promising method for characterizing microstructural changes or differences with neuropathology and treatment.
The broad spectrum of MR contrast mechanisms makes MRI one of the most powerful and flexible imaging tool for diagnosis in the CNS. Measurement of the signal attenuation from water diffusion is one of the most important contrast mechanisms. In particular, diffusion tensor imaging (DTI) may be used to map and characterize the three-dimensional diffusion of water as a function of spatial location.1,2 The diffusion tensor describes the magnitude, the degree of anisotropy, and the orientation of diffusion anisotropy. Estimates of white matter connectivity patterns in the brain from white matter tractography may be obtained using the diffusion anisotropy and the principal diffusion directions.3-5
Many developmental, aging and pathologic processes of the CNS influence the microstructural composition and architecture of the affected tissues. The diffusion of water within the tissues will be altered by changes in the tissue microstructure and organization; consequently, diffusion-weighted (DW) MRI methods including DTI are potentially powerful probes for characterizing the effects of disease and aging on microstructure. Indeed, the applications of DTI are rapidly increasing because the technique is highly sensitive to changes at the cellular and microstructural level.
--------------------------
Results
Being HIV positive was associated with greater decreases in executive function and global cognition.
Additionally, using DTI, we found that the HIV-positive group had a greater increase in mean diffusivity, but we did not find group differences in volume change using VBM.
With respect to the HIV status by age group interaction, this was statistically significant for change in global cognition, with older HIV-positive individuals showing greater global cognitive decline, but there were no significant interaction effects on other measures.
Lastly, change in cognitive performance was correlated with change in the DTI measures, and this effect was stronger for the HIV-positive participants.
Conclusions
In the present study, we found some evidence for accelerated ageing in HIV-positive individuals, with a statistically significant HIV status by age group interaction in global cognition, although this interaction could not be explained by the imaging findings. Moreover, we also found that change in cognitive performance was correlated with change in the DTI measures, and this effect was stronger for the HIV-positive participants. This will need replication in larger studies using a similarly lengthy follow-up period.
Group difference analysis for the neuropsychological assessment
HIV status
There was a statistically significant effect of HIV status on change in executive function [F(1,55) = 6.77; P = 0.012; d = 0.70] and global cognition [F(1,55) = 7.89; P = 0.007; d = 0.76]. The results suggest that the HIV-positive group (executive function: mean -0.40; SD 0.75; global cognition: mean -0.12; SD 0.33) displayed a greater reduction in z-scores than the seronegative controls (executive function: mean 0.06; SD 0.44; global cognition: mean 0.08; SD 0.19). Differences were nonsignificant for the other cognitive domains [in each of these, F(1,55) ≤ 2.71; P ≥ 0.106; d ≤ 0.45].
Age
There was a statistically significant effect of age group on change in complex attention [F(1,55) = 6.70; P = 0.011; d = 0.72], perceptual motor function [F(1,55) = 5.83; P = 0.020; d = 0.66] and global cognition [F(1,55) = 6.21; P = 0.016; d = 0.68]. Here, the older participants showed a greater reduction in z-scores than the younger participants. The effect of age was not significant for the other cognitive domains [in each of these, F(1,55) ≤ 2.41; P ≥ 0.127; d ≤ 0.36]. Critically, this included executive function, which was the only domain to show an effect of HIV status.
HIV status by age group interaction
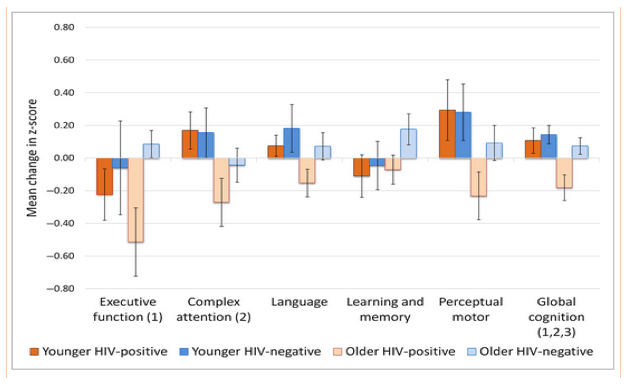
One important question was whether the results were suggestive of accelerated ageing with HIV infection. Here, we were interested in whether there was a significant HIV status by age group interaction. This was only significant for change in global cognition [F(1,55) = 4.39; P = 0.041], where there was a larger difference in global change scores between the older HIV-positive and HIV-negative participants [t(31) = 3.25; P = 0.003; d = 1.17] than between the younger participants [t(24) = 0.54; P = 0.598; d = 0.23]. These cognitive findings are summarized in Fig. 1.
Neuroimaging findings -
Age
In the VBM analysis, there was no effect of age on rate of change in regional grey matter volume (family-wise error-corrected P < 0.05). With respect to the DTI measures, older participants showed a greater decrease in FA than the younger group in the right anterior corona radiata, right anterior limb of the internal capsule, and the right genu and splenium of the corpus callosum. There were also significant age effects on change MD, in widespread regions bilaterally (Fig. 3).
Figure 1.
Mean (standard error) change in z-score for each cognitive domain. Change scores were generated using baseline minus follow-up. Negative values therefore represent a decline in performance while positive values represent an improvement. (1) Main effect of HIV status; (2) Main effect of age group; (3) HIV status by age group interaction (P < 0.05) .
Introduction
HIV-1 infection is characterized by inflammation of the central nervous system (CNS) [1]. Following the introduction of highly active antiretroviral therapy (HAART), the prognosis of HIV-positive individuals in terms of morbidity and mortality has greatly improved [2]. However, a substantial proportion of patients report mild cognitive problems, despite being on treatment (for a review, see [3]), with reported prevalence of HIV-associated neurocognitive disorders in 30-50% of individuals [4, 5]. This suggests the possibility that there is HIV-related neuronal damage even in the subclinical stages of infection in people who are on stable HAART.
With improved survival following HIV infection, one in four individuals living with HIV in the UK is now aged ≥ 50 years [6]. The combined influence of ageing and HIV infection is an important research area as there is evidence that HIV infection is associated with a higher prevalence of age-related conditions at a younger age [7].
Moreover, it has been suggested that older age is associated with an increased risk of HIV-associated neurocognitive disorders [8-10], indicating a possible effect of accelerated neurocognitive ageing (see [11]). This was addressed in a recent review of behavioural and neuroimaging studies published between 2011 and 2014 [12]. This revealed mixed findings, with 11 studies (six behavioural and five neuroimaging) supportive of the accelerated ageing hypothesis in HIV infection, and nine studies (four behavioural and five neuroimaging) not supportive. The authors suggested that methodological differences could explain the discrepancies, with studies that used global cognitive measures or neuroimaging techniques with poor specificity being less likely to find an interaction between HIV infection and age. However, as the majority (19 out of 20) of these studies were cross-sectional, this limited the extent to which accelerated neurocognitive ageing could be ascertained.
There have been fewer longitudinal studies that have investigated the impact of age and HIV infection on change in cognitive performance, but there is evidence for interacting effects of HIV infection and ageing. In one study, older HIV-positive individuals showed a greater decline in executive function over 5 years than younger HIV-positive individuals, whereas there was no effect of age on longitudinal performance for the HIV-seronegative controls [13]. Similarly, individuals with HIV infection showed a greater 1-year decline in verbal memory with increasing age, whereas the seronegative controls showed stable or improved performance with age [14]. However, other longitudinal studies have not found interactive effects of HIV infection and age on cognitive change. For example, although HIV infection was associated with faster rates of cognitive decline in executive function, there was no evidence for accelerated decline with older age [15]; and age was not a predictor of cognitive decline in an HIV-infected cohort assessed regularly for up to 5 years [16]. Instead, change in cognitive status was associated with disease severity, race, premorbid intelligence, current depressive symptoms, lifetime psychiatric diagnoses, and non-HIV-related comorbidities. Although this latter study highlighted the multifaceted risk factors for cognitive decline in HIV infection, it did not determine the degree to which HIV itself is linked to cognitive change in the absence of other risk factors.
Magnetic resonance imaging (MRI) studies have further explored the impact of prolonged HIV exposure on brain structure. One such study found a greater increase in mean diffusivity (MD) in HIV-positive individuals compared with seronegative controls; however, the influence of age on HIV-associated change in MD was not explored. The trajectories of volume change in HIV-positive individuals and healthy controls have also been investigated [15]. Here, the HIV-positive group showed significantly greater change per year of infection than controls in terms of hippocampal and insula atrophy and significantly increased lateral ventricle volumes. There was also significant acceleration of age-related trajectories of grey matter volume change in the thalamus and frontal, sensorimotor, and temporo-parietal neocortical regions.
In a previous study [17], we examined HIV-positive and HIV-negative older and younger individuals. All participants were asymptomatic with undetectable HIV viral loads, without medical or psychiatric comorbidity, or alcohol or substance misuse, and they had all been stable on HAART for at least 6 months prior to enrolment in the study. Comparison of the HIV groups did not show significant differences on the neuropsychological tests after Bonferroni correction. However, we found reduced grey matter volume on MRI in our HIV-positive participants. Moreover, on fluorodeoxyglucose-positron emission tomography (FDG-PET) and MRI-based arterial spin labelling, [18] we found age-related reductions in the metabolic rate of glucose consumption and cerebral blood flow in frontal brain regions, and consistent (although small) reductions in the anterior cingulate in HIV-positive individuals. Across all measures, there were no significant HIV status by age group interactions.
In the present study, we re-assessed these participants, extending previous longitudinal investigations in the following ways: (1) all were on HAART, and all had undetectable viral load at baseline; (2) all were Caucasian men who have sex with men; (3) other confounding variables were controlled; and (4) mean follow-up duration was 4.2 years. We investigated the interaction of the effects of HIV status and age on individual cognitive domains and global cognitive performance and we related these findings to concurrent MRI measures to see whether any cognitive deterioration was accompanied by associated changes in structural brain metrics. We hypothesized that:
1. there would be a significant HIV status by age group interaction in terms of change scores on cognitive testing;
2. there would be a significant HIV status by age group interaction in terms of change in neuroimaging indexes;
3. there would be significant correlations between cognitive and neuroimaging changes.
Methods
Participant population
Fifty-five participants (67%) from our previous cross-sectional study [17, 18] were included in the present study, with a mean time between assessments of 4.2 years [standard deviation (SD) 0.8 years]. Informed consent was obtained from all participants according to the Declaration of Helsinki and the study was approved by the NHS research ethics committee East London Research Ethics Committee 3 (Ref. 11/LO/0037).
All participants were Caucasian and self-identified as 'men who have sex with men'.
Baseline exclusion criteria were hepatitis B or C virus infection, any confounding neurological disorder, a history of head trauma with loss of consciousness > 10 min, and a history of harmful alcohol (> 25 units of alcohol per week) or substance misuse. Additionally, the HIV-positive participants were stable on HAART with an undetectable viral load (< 50 HIV-1 RNA copies/mL) and did not have a current or previous CNS AIDS condition. These criteria were met at follow-up, although one HIV-positive individual reported harmful alcohol use and another had a viral load of 147 copies/mL. Excluding these two individuals did not influence the interpretation of any results; therefore, analyses based on the full sample are reported.
Medical and psychiatric evaluation
Participants underwent a brief medical assessment and routine blood investigations which included determination of HIV viral load and CD4 count (HIV-positive group), conformation of HIV-negative status (control group), syphilis screen, hepatitis B and C virus, renal and liver function tests, assessment of bone and lipid profiles, and vitamin B12 measurement. The Beck Depression Inventory [19], Beck Anxiety Inventory [20] and Profile of Mood States [21] were used to assess mood state. The frequency of perceived memory difficulties was evaluated using the Prospective and Retrospective Memory Questionnaire [22].
Cognitive assessment
A wide range of cognitive tests were administered by trained psychologists (SC and BH) using standardized procedures. Raw scores were converted to z-scores using baseline seronegative findings, which were then averaged to form five cognitive domain scores: executive function, complex attention, learning and memory, language, and perceptual motor function. The tasks that were included in each domain can be found in Table 1. Change scores were generated by subtracting the baseline from follow-up z-scores.
Results
Clinical and psychiatric evaluation
Table 2 shows the characteristics of study participants. The younger HIV-positive participants were significantly older than the younger HIV-seronegative group. No other differences were evident between these younger groups. The older HIV-positive participants did not differ from the older HIV-seronegative group. In particular, there were no group differences in anxiety or depression measures in either the younger or older participants. Looking at HIV-related variables, the older and younger HIV-positive individuals did not differ significantly on current [t(30) = 0.43; P = 0.673; d = 0.16] or nadir [t(28) = 0.49; P = 0.628; d = 0.19] CD4 count. The older HIV-positive participants had a longer average number of years since diagnosis than the younger participants [t(28) = 3.26; P = 0.003; d = 1.19], but the difference in treatment duration did not reach statistical significance [t(28) = 1.97; P = 0.059; d = 0.72].
Group difference analysis for the neuropsychological assessment
HIV status
There was a statistically significant effect of HIV status on change in executive function [F(1,55) = 6.77; P = 0.012; d = 0.70] and global cognition [F(1,55) = 7.89; P = 0.007; d = 0.76]. The results suggest that the HIV-positive group (executive function: mean -0.40; SD 0.75; global cognition: mean -0.12; SD 0.33) displayed a greater reduction in z-scores than the seronegative controls (executive function: mean 0.06; SD 0.44; global cognition: mean 0.08; SD 0.19). Differences were nonsignificant for the other cognitive domains [in each of these, F(1,55) ≤ 2.71; P ≥ 0.106; d ≤ 0.45].
Age
There was a statistically significant effect of age group on change in complex attention [F(1,55) = 6.70; P = 0.011; d = 0.72], perceptual motor function [F(1,55) = 5.83; P = 0.020; d = 0.66] and global cognition [F(1,55) = 6.21; P = 0.016; d = 0.68]. Here, the older participants showed a greater reduction in z-scores than the younger participants. The effect of age was not significant for the other cognitive domains [in each of these, F(1,55) ≤ 2.41; P ≥ 0.127; d ≤ 0.36]. Critically, this included executive function, which was the only domain to show an effect of HIV status.
HIV status by age group interaction
One important question was whether the results were suggestive of accelerated ageing with HIV infection. Here, we were interested in whether there was a significant HIV status by age group interaction. This was only significant for change in global cognition [F(1,55) = 4.39; P = 0.041], where there was a larger difference in global change scores between the older HIV-positive and HIV-negative participants [t(31) = 3.25; P = 0.003; d = 1.17] than between the younger participants [t(24) = 0.54; P = 0.598; d = 0.23]. These cognitive findings are summarized in Fig. 1.
Neuroimaging findings
HIV status
Using VBM, no regions reached family-wise error-corrected significance for the main effect of HIV status on rate of grey matter volume change. On the DTI metrics, there was no significant main effect of HIV status on change in FA. There was, however, a significant difference for change in MD, with the HIV-positive group showing a greater increase in MD than the seronegative controls in the corpus collosum, the right posterior corona radiata and right posterior thalamic radiation (Fig. 2).
Age
In the VBM analysis, there was no effect of age on rate of change in regional grey matter volume (family-wise error-corrected P < 0.05). With respect to the DTI measures, older participants showed a greater decrease in FA than the younger group in the right anterior corona radiata, right anterior limb of the internal capsule, and the right genu and splenium of the corpus callosum. There were also significant age effects on change MD, in widespread regions bilaterally (Fig. 3).
HIV status by age group interaction
No significant interactions were identified between HIV status and age group for change in grey matter volume, change in FA, or change in MD.
Correlations between HIV, neuroimaging, and neuropsychological variables
To allow comparison between the neuropsychological and neuroimaging variables, atlas-based regions of interest (frontal white matter and the genu, body, and splenium of the corpus callosum) were created in fsl using the JHU ICBM-DTI-81 White-Matter Labels Atlas ( http://cmrm.med.jhmi.edu). FA and MD values were extracted from the baseline and follow-up scans and were then subtracted to generate FADIFF (change in FA) and MDDIFF (change in MD) scores.
A series of hierarchical multiple regression models were used to explore the influence of HIV status and FADIFF or MDDIFF on global cognitive change. Step 1 adjusted for the covariates of age group, IQ, and time between assessments. At step 2, the primary effects of HIV status and FADIFF or MDDIFF were added, and at step 3, an HIV status x FADIFF/MDDIFF cross-product interaction term was entered. Here, we were interested in whether the interaction term significantly added to the variance (Δr2) explained in global cognitive change, which would suggest that the strength of the association between change in the DTI metrics and change in cognition varied as a function of HIV status.
The results of the hierarchical regressions can be found in Table 3. Adding HIV status and FADIFF or MDDIFF added to the prediction of global cognitive change, with shared variances ranging from 26% to 37%. Importantly, at step 3, the HIV status x FADIFF or HIV status x MDDIFF interaction terms were significant for the majority of regions (all except FADIFF in the body of the corpus callosum). The interaction explained a further 7-15% of the variance in global cognitive change. This association was further explored separately in the patient and control groups. In the HIV-positive group, worsening cognitive performance was associated with a greater increase in MD (β ≤ -0.531; P < 0.01) and a greater decrease in FA (β ≥ 0.483; P < 0.01), whereas these relationships were of a smaller magnitude and were nonsignificant in the seronegative group (MD: β ≥ -0.214; P ≥ 0.442; FA: β ≤ 0.429; P ≥ 0.060). Lastly, in the HIV-positive participants we assessed whether the association between change in DTI metrics and change in cognitive performance was modified by age group. The age by DTI metric cross-product interaction was nonsignificant for all measures.
Discussion
This study investigated longitudinal change in cognitive performance and quantitative MRI findings in HIV-positive individuals. We hypothesized that there would be an interaction between age group and HIV status, supporting the notion of accelerated ageing in HIV infection [11]. In our small sample, we found that the HIV-positive participants had a greater global cognitive decline than their seronegative controls and this was exacerbated in the older age group. However, when the cognitive domains were analysed separately, there were no significant HIV status by age group interactions. On neuroimaging measures, HIV infection and older age were each associated with a greater increase in MD, but there were no significant HIV status by age group interactions. Lastly, change in cognitive performance correlated with change in DTI measures, and this effect was stronger for the HIV-positive participants but did not differ between the age groups.
It is of note that HIV status and age group appeared to affect different components of cognitive function. There was a statistically significant effect of HIV on executive function, whereas age group influenced domains that involved speed of performance (i.e. change in complex attention and perceptual motor function). With respect to executive function, younger HIV-positive individuals, as well as older ones, showed worse performance (in terms of change scores) relative to controls. This suggests that it was HIV infection itself that influenced this domain rather than an effect of age group. While the present results are broadly consistent with previous neuropsychological findings (e.g. [15]), we must acknowledge that caution is needed in interpreting these cognitive findings because of the small sample size in our groups.
The VBM results showed no influence of HIV status or age group on grey matter volume, and no interaction between HIV status and age group, suggesting that the groups showed an equivalent rate of change in volume over time. At baseline, we found reduced grey matter volume in a cluster encompassing the medial and superior frontal gyrus in the HIV-positive participants [17]. Longitudinally, we did not find evidence that this had progressed (although this might perhaps have reflected the relatively young age of our older group, with a minimum age of 50 years at baseline). One possible interpretation of this particular finding would be that HIV has an effect on grey matter volume early in the disease process, for example pretreatment. By contrast, one previous study [15] did find evidence of HIV status by age effects on regional grey matter in a sample that was larger than ours, but less highly selected to exclude confounding factors.
Our DTI findings highlighted the importance of longitudinal evaluation in HIV infection, as baseline analysis in this sample did not find any differences on either MD or FA [17], but in the present analysis, we found that both HIV infection and age group were associated with a greater longitudinal increase in MD. In other words, HIV infection was associated with greater cerebral white matter damage through time, even in patients who had a good treatment response to HAART and no significant cerebrovascular risk factors.
This may reflect persistent immune activation and neuro-inflammation [32, 33]. The present results also suggest that the group differences in DTI change scores might be clinically relevant, as regression analysis indicated that cognitive performance changed in conjunction with DTI change, and that this association was stronger for the HIV-positive group. Thus, the HIV-positive patients with the greatest increase in MD, or decrease in FA, showed the greatest decline in cognitive performance (in terms of change scores). There have been few other longitudinal DTI studies in persons with HIV infection, but our results are in line with a previous study that showed an HIV-related increase in MD in the genu of the corpus callosum and a correlation between change in global cognition and DTI measures in the corpus callosum in HIV-positive individuals [34]. We have extended this from 1 year to a longer follow-up duration and, using voxel-based procedures, we identified more widespread longitudinal changes. We also showed that older age was associated with a greater increase in MD and decrease in FA. However, in the present sample, there were no HIV status by age group interactions, and similar DTI-cognitive associations were evident in the younger and older HIV-positive participants.
Our study had a number of strengths. It had a relatively long follow-up, with a mean duration of 4.2 years. It was well controlled in terms of comorbidities, and drug and alcohol use, and the HIV-positive group was asymptomatic and stable on HAART. We also carefully recruited a control group with a similar sociodemographic background who were well matched on age, IQ, and education. We can, therefore, be confident that the findings are likely to be the result of HIV infection and not of other confounding factors. There were, however, some limitations. Sample sizes were relatively small as we were limited by the size of the original sample. Moreover, the detail of our neuropsychological assessment, the range of our imaging protocols (which included positron emission tomography at baseline), and the duration of our follow-up may have affected the attrition rate, which was imbalanced across the groups. The findings should be corroborated in larger samples, allowing for variability in other important factors that can affect cognition such as anxiety and depression scores.
In conclusion, we found independent (main) effects of both HIV status and age group on longitudinal change in cognitive performance and change through time in mean diffusivity using DTI, but not an effect on volumetrics. The results suggested accelerated ageing in HIV infection, in that there was a significant HIV status by age group interaction for change in global cognition. Being HIV positive and older was associated with a worsening in overall cognitive performance. However, this interaction could not be explained by the imaging findings, as there were no significant interactions for any of the imaging metrics. Further work is needed to look at other biological factors that could explain change in overall cognitive performance in HIV infection, including metabolic findings and treatment effects.
|
|
| |
| |
|
|
|