 |
 |
 |
| |
Acceptability of long-acting injectable cabotegravir (CAB LA) in HIV-uninfected individuals: HPTN 077
|
| |
| |
High Acceptability of Injected Cabotegravir in Trial, But More Future Interest Outside US
HIV Research for Prevention (HIVR4P), October 21-25, 2018, Madrid
Mark Mascolini
People getting injections of long-acting cabotegravir (CAB LA) in the HPTN 077 clinical trial found the PrEP candidate highly acceptable by several measures [1]. But trial participants outside the United States had more interest in future injectable PrEP use.
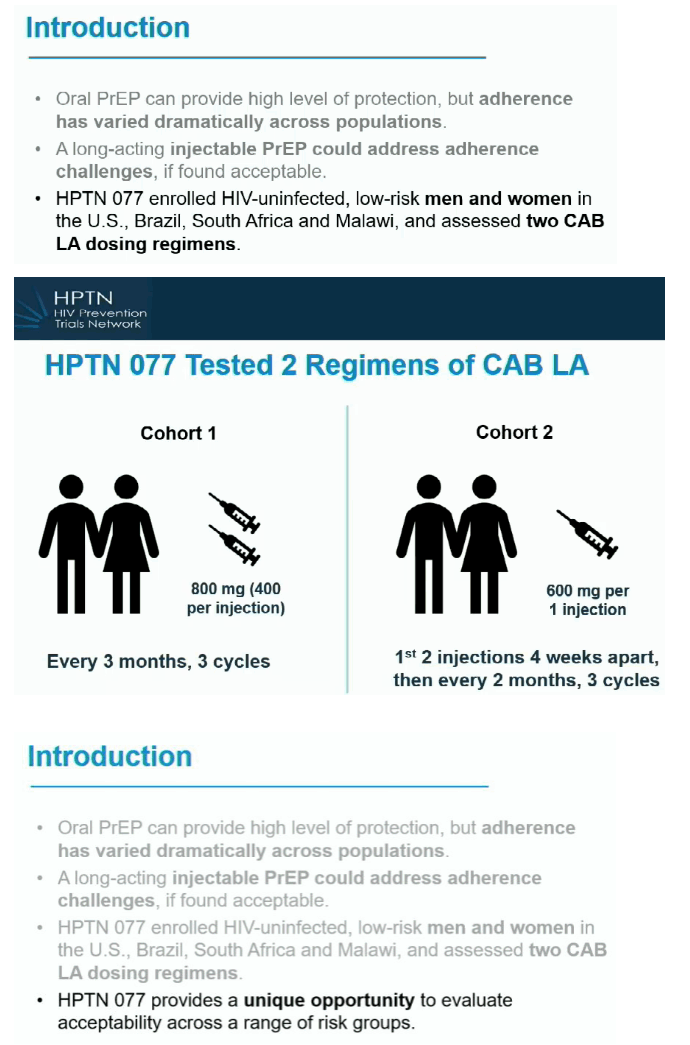
Long-acting preexposure prophylaxis (PrEP) holds the promise of greater convenience and possibly adherence for people who prefer injections every few months over daily pills. HPTN 077 was a double-blind placebo-controlled trial testing 2 doses of the integrase inhibitor CAB LA in people at low risk for HIV infection in the United States, Brazil, South Africa, and Malawi. Primary results established that an intramuscular dose of 600 mg every 8 weeks met drug level targets for women and men [2].
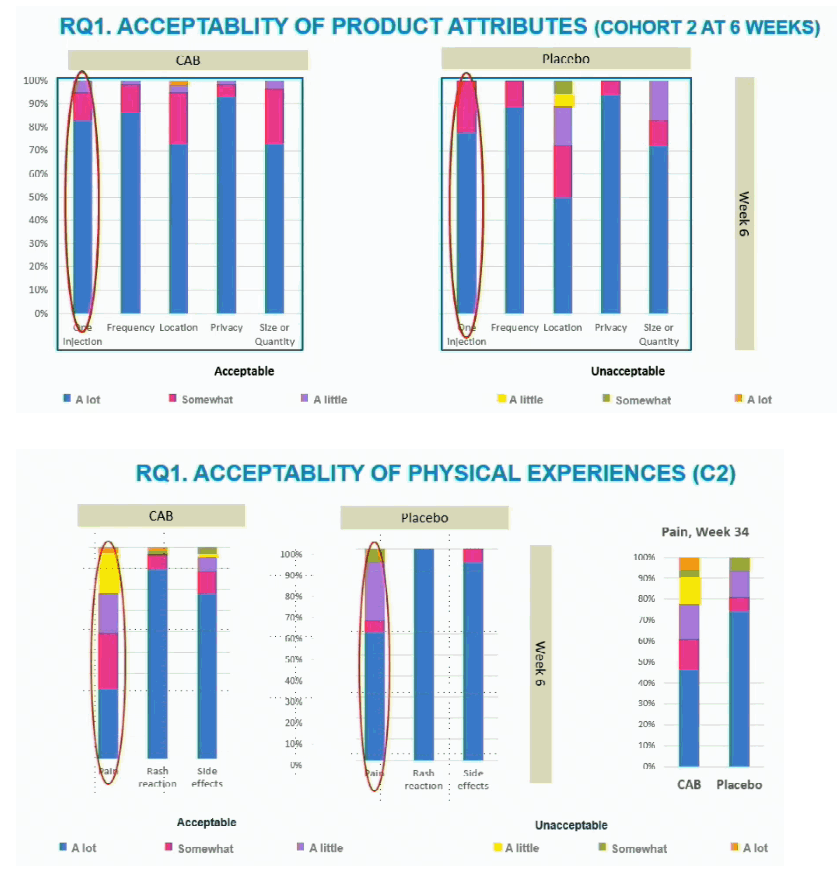
This analysis assessed acceptability of CAB LA and future interest in this PrEP strategy among trial participants. The study focused on trial cohort 2, who received a 4-week CAB oral loading dose, two 600-mg CAB LA injections 4 weeks apart, then subsequent injections every 2 months. Researchers determined acceptability of 8 CAB LA characteristics: dosing, quantity, injection site, pain, rash, side effects, privacy, and schedule. They assessed injection site reactions throughout the study and determined participant preferences for injectable versus other prevention products. The investigators weighed future interest in injectable PrEP through 6 items about commitment to use.
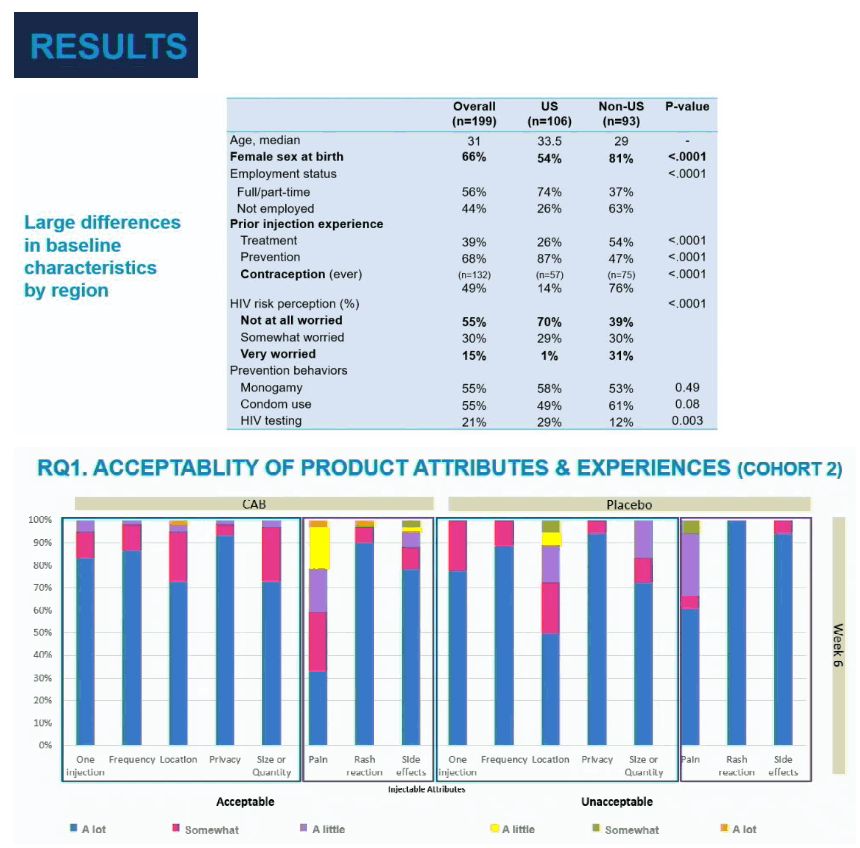
Among all 199 trial participants, the 106 people from the United States included a significantly lower proportion of women than the 93 non-US participants (54% versus 81%, P < 0.0001). The US contingent included a higher proportion not worried at all about HIV risk (70% versus 39%) and a lower proportion very worried about HIV (1% versus 31%) (P < 0.0001). People in the United States were more likely to have a job than non-US participants (74% versus 37%, P < 0.0001).
At week 6 in cohort 2 more than 70% of participants stated high acceptability of 7 of 8 CAB LA characteristics (listed above). The exception was pain, for which about 30% stated high acceptability, about 30% moderate acceptability, and the rest lower levels of acceptability. In contrast, 60% of placebo recipients gave a high acceptability rating for pain. At study week 34 proportions of CAB LA and placebo recipients with high acceptability of pain were about 45% versus 75%. Almost 90% of CAB and placebo recipients found the dosing scheduled highly acceptable throughout the study.
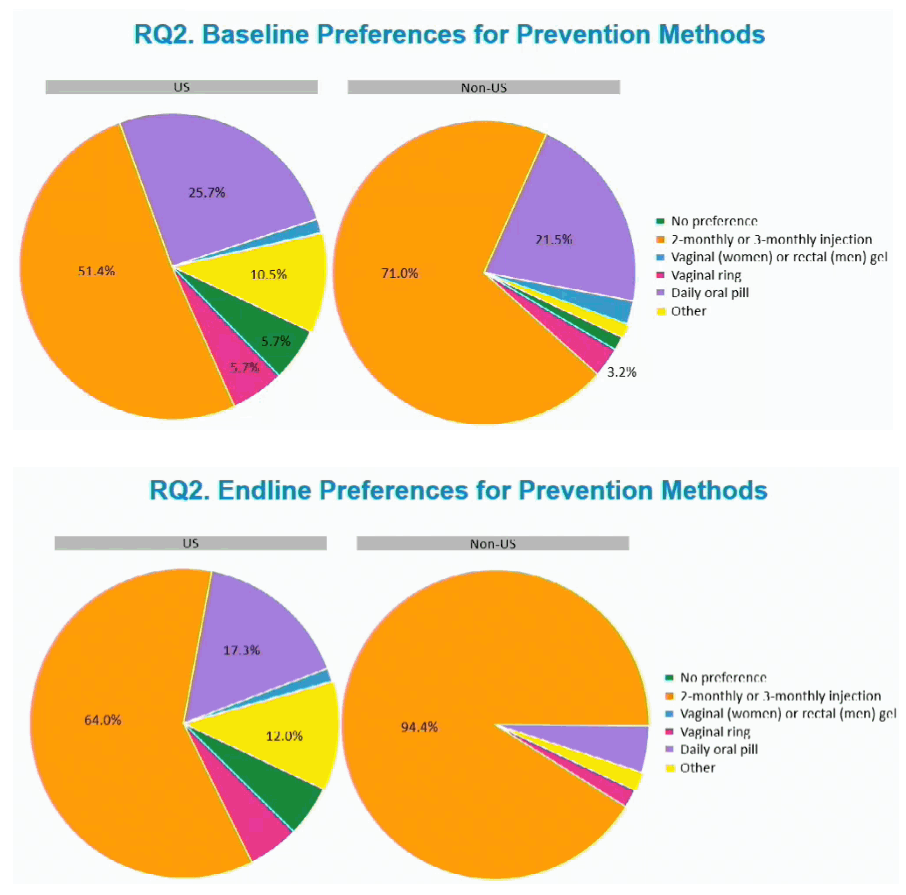
When the trial began 51.4% of US participants versus 71% of non-US participants favored 2- or 3-monthly injections as their preferred HIV prevention method. The second most popular prevention method at the baseline visit was a daily pill: 25.7% in the US and 21.5% outside the US. At the end of the study the proportion favoring 2- or 3-monthly injections rose substantially in both the US (to 64%) and outside the US (94.4%). At that point the proportion favoring a daily pill had dwindled to 17.3% in the US and to about 5% outside the US.
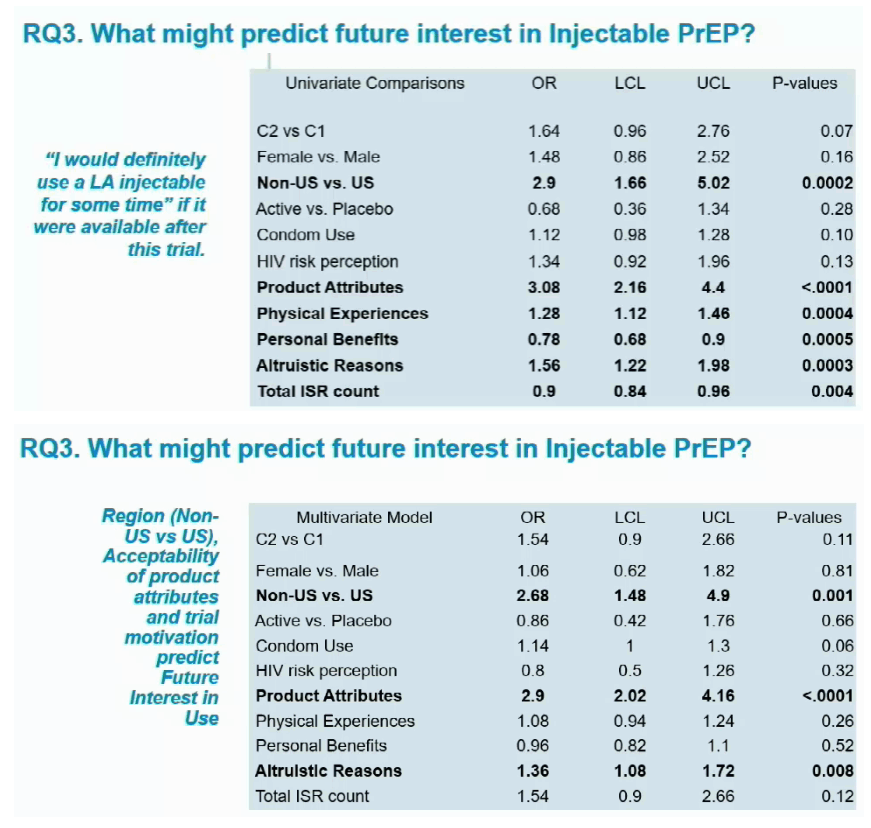
Longitudinal ordinal logistic regression analysis identified 3 factors independently associated with future interest in injectable PrEP. Non-US participants had more than twice higher odds of favoring injectable PrEP in the future than US participants (odds ratio [OR] 2.68, confidence limits [CL] 1.48 to 4.9, P = 0.001). People who found product attributes acceptable had almost tripled odds of favoring future injectable PrEP (OR 2.9, CL 2.02 to 4.16, P < 0.0001). And those who entered the trial for altruistic reasons (versus personal benefits or money) had about one third higher odds of favoring future injectable PrEP (OR 1.36, CL 1.08 to 1.72, P = 0.008). Gender, HIV risk perception, and total injection site reactions did not affect future interest in injectable PrEP.
HPTN 077 investigators cautioned that people in this trial had a low risk for HIV infection, and acceptability may differ in people at higher risk and those who use injectable PrEP longer. The researchers stressed their finding of a strong preference for long-term injection as a PrEP strategy both when the trial began and at its end. Most participants randomized to CAB LA reported injection pain but usually described it as acceptable.
References
1. Tolley E, Zangeneh SZ, Chau G, et al. Acceptability of long-acting injectable cabotegravir (CAB LA) in HIV-uninfected individuals: HPTN 077. HIV Research for Prevention (HIVR4P), October 21-25, 2018, Madrid. Abstract OA05.01.
2. Landovitz R, Li S, Grinsztejn B, et al. Safety, tolerability and pharmacokinetics of long-acting injectable cabotegravir in low-risk HIV-uninfected women and men: HPTN 077. IAS 2017. Paris. Abstract TUAC0106LB. http://www.natap.org/2017/IAS/IAS_33.htm
WEBCAST: http://webcasts.hivr4p.org/console/player/40334?mediaType=slideVideo&&crd_fl=1&ssmsrq=1540472689710&ctms=5000&csmsrq=1077



|
| |
|
 |
 |
|
|